预约演示
更新于:2026-03-01
TD-01
更新于:2026-03-01
概要
基本信息
原研机构 |
在研机构- |
非在研机构 |
权益机构- |
最高研发阶段终止临床前 |
首次获批日期- |
最高研发阶段(中国)- |
特殊审评- |
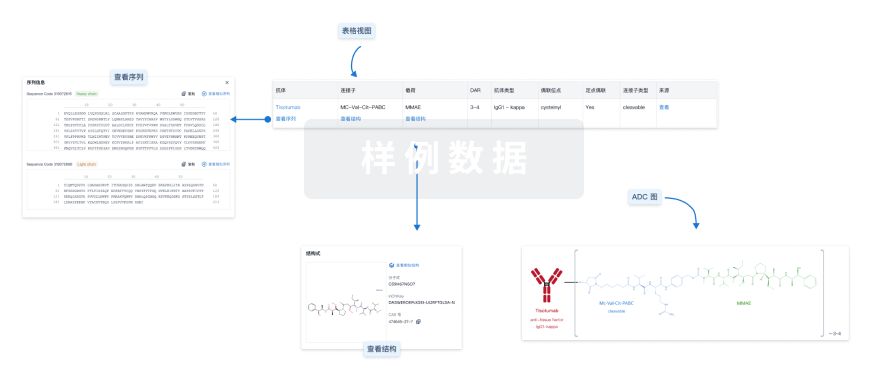
结构/序列
使用我们的ADC技术数据为新药研发加速。
登录
或
关联
100 项与 TD-01 相关的临床结果
登录后查看更多信息
100 项与 TD-01 相关的转化医学
登录后查看更多信息
100 项与 TD-01 相关的专利(医药)
登录后查看更多信息
5
项与 TD-01 相关的文献(医药)2022-09-01·Environmental research2区 · 环境科学与生态学
Transcriptome profiling reveals upregulation of benzoate degradation and related genes in Pseudomonas aeruginosa D6 during textile dye degradation
2区 · 环境科学与生态学
Article
作者: Pandita, Priti Raj ; Patel, Amrutlal K ; Patel, Zarna ; Nanjani, Sandhya ; Joshi, Madhvi N ; Joshi, Chaitanya ; Sharma, Shruti ; Pandit, Ramesh
An upsurge in textile dye pollution has demanded immediate efforts to develop an optimum technology for their bioremediation. However, the molecular mechanism underpinning aerobic decolorization of dyes is still in its infancy. Thus, in the current work, the intricacies of aerobic remediation of textile dyes by Pseudomonas aeruginosa D6 were understood via a transcriptomic approach. The bacterium isolated from the sludge sample of a common effluent treatment plant was able to decolorize 54.42, 57.66, 50.84 and 65.86% of 100 mg L-1 of four different dyes i.e., TD01, TD04, TD05, and TD06, respectively. The maximum decolorization was achieved within six days and thus, the first and sixth day of incubation were selected for transcriptome analysis at the early and late phase of the decolorization, respectively. The expression profiles of all samples were compared to gain insight into the dye-specific response of bacterium and it was found that it behaved most uniquely in the presence of the dye TD01. Several genes critical to core metabolic processes like the TCA cycle, glycolysis, pentose phosphate pathway, translation, cell motility etc. Were found to be overexpressed in the presence of dyes. Interestingly, in response to dyes, the benzoate degradation pathway was significantly upregulated in the bacterium as compared to control (i.e., bacterium without dye). Thus, seven genes contributing to the induction of the same were further studied by RT-qPCR analysis. Overall, the involvement of the benzoate pathway implies the appearance of aromatic intermediates during decolorization, which in turn infers dye degradation.
2020-06-01·Journal of biotechnology3区 · 工程技术
Substrate profiling and tolerance testing of Halomonas TD01 suggest its potential application in sustainable manufacturing of chemicals
3区 · 工程技术
Article
作者: Wang, Zhiwen ; Jin, Biao ; Mao, Yufeng ; Guo, Yanmei ; Zhang, Xin ; Zhang, Jing ; Chen, Tao
Halomonas TD01, which can grow under non-sterile and continuous processes at high pH and high salt concentrations, is a robust platform for PHA production from glucose. For extending other low-cost sustainable substrates and increasing the potential application in other value-added products, a better understanding of substrates utilization and chemicals tolerance is necessary. In this study, the substrate profiling of TD01 was analyzed via Biolog. Phenotype microarray results demonstrated that TD01 has a wide-ranging substrate spectrum and can utilize 140 of the 190 test compounds. Some cheap, abundant carbon sources, such as sodium acetate, glycerol, ethanol and lactate can well support the growth of TD01 in shake-flask, and are therefore suggested to be its alternative low-cost substrates for chemicals production in future. Furthermore, the tolerance of TD01 to various chemicals was tested. The results showed that the tolerability of TD01 to high concentrations of organic acid salts is prominent. When adding 75 g/L sodium acetate, 100 g/L succinic acid and 100 g/L itaconic acid in the medium, the growth rate reduced 56.14%, 52.63% and 47.37%, respectively. All these results highlight TD01 as a promising, next generation industrial workhorse in chemicals biomanufacturing from cheap organic acid salts.
2011-12-01·Microbial cell factories2区 · 工程技术
Comparative genomics study of polyhydroxyalkanoates (PHA) and ectoine relevant genes from Halomonas sp. TD01 revealed extensive horizontal gene transfer events and co-evolutionary relationships
2区 · 工程技术
ArticleOA
作者: Aibaidula, Gulsimay ; Chen, Jin-Chun ; Cai, Lei ; Dong, Xin-Ran ; Chen, Guo-Qiang ; Tan, Dan ; Tian, Wei-Dong
Abstract:
Background:
Halophilic bacteria have shown their significance in industrial production of polyhydroxyalkanoates (PHA) and are gaining more attention for genetic engineering modification. Yet, little information on the genomics and PHA related genes from halophilic bacteria have been disclosed so far.
Results:
The draft genome of moderately halophilic bacterium, Halomonas sp. TD01, a strain of great potential for industrial production of short-chain-length polyhydroxyalkanoates (PHA), was analyzed through computational methods to reveal the osmoregulation mechanism and the evolutionary relationship of the enzymes relevant to PHA and ectoine syntheses. Genes involved in the metabolism of PHA and osmolytes were annotated and studied in silico. Although PHA synthase, depolymerase, regulator/repressor and phasin were all involved in PHA metabolic pathways, they demonstrated different horizontal gene transfer (HGT) events between the genomes of different strains. In contrast, co-occurrence of ectoine genes in the same genome was more frequently observed, and ectoine genes were more likely under coincidental horizontal gene transfer than PHA related genes. In addition, the adjacent organization of the homologues of PHA synthase phaC1 and PHA granule binding protein phaP was conserved in the strain TD01, which was also observed in some halophiles and non-halophiles exclusively from γ-proteobacteria. In contrast to haloarchaea, the proteome of Halomonas sp. TD01 did not show obvious inclination towards acidity relative to non-halophilic Escherichia coli MG1655, which signified that Halomonas sp. TD01 preferred the accumulation of organic osmolytes to ions in order to balance the intracellular osmotic pressure with the environment.
Conclusions:
The accessibility of genome information would facilitate research on the genetic engineering of halophilic bacteria including Halomonas sp. TD01.
100 项与 TD-01 相关的药物交易
登录后查看更多信息
研发状态
10 条进展最快的记录, 后查看更多信息
登录
| 适应症 | 最高研发状态 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|---|
| 三阴性乳腺癌 | 临床前 | 韩国 | 2024-07-21 |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
No Data | |||||||
登录后查看更多信息
转化医学
使用我们的转化医学数据加速您的研究。
登录
或

药物交易
使用我们的药物交易数据加速您的研究。
登录
或

核心专利
使用我们的核心专利数据促进您的研究。
登录
或

临床分析
紧跟全球注册中心的最新临床试验。
登录
或

批准
利用最新的监管批准信息加速您的研究。
登录
或

生物类似药
生物类似药在不同国家/地区的竞争态势。请注意临床1/2期并入临床2期,临床2/3期并入临床3期
登录
或

特殊审评
只需点击几下即可了解关键药物信息。
登录
或

生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用