预约演示
更新于:2025-11-15
IONIS-DNM2-2.5Rx
更新于:2025-11-15
概要
基本信息
在研机构- |
非在研机构 |
最高研发阶段终止临床1/2期 |
首次获批日期- |
最高研发阶段(中国)- |
特殊审评- |
登录后查看时间轴
结构/序列
使用我们的RNA技术数据为新药研发加速。
登录
或
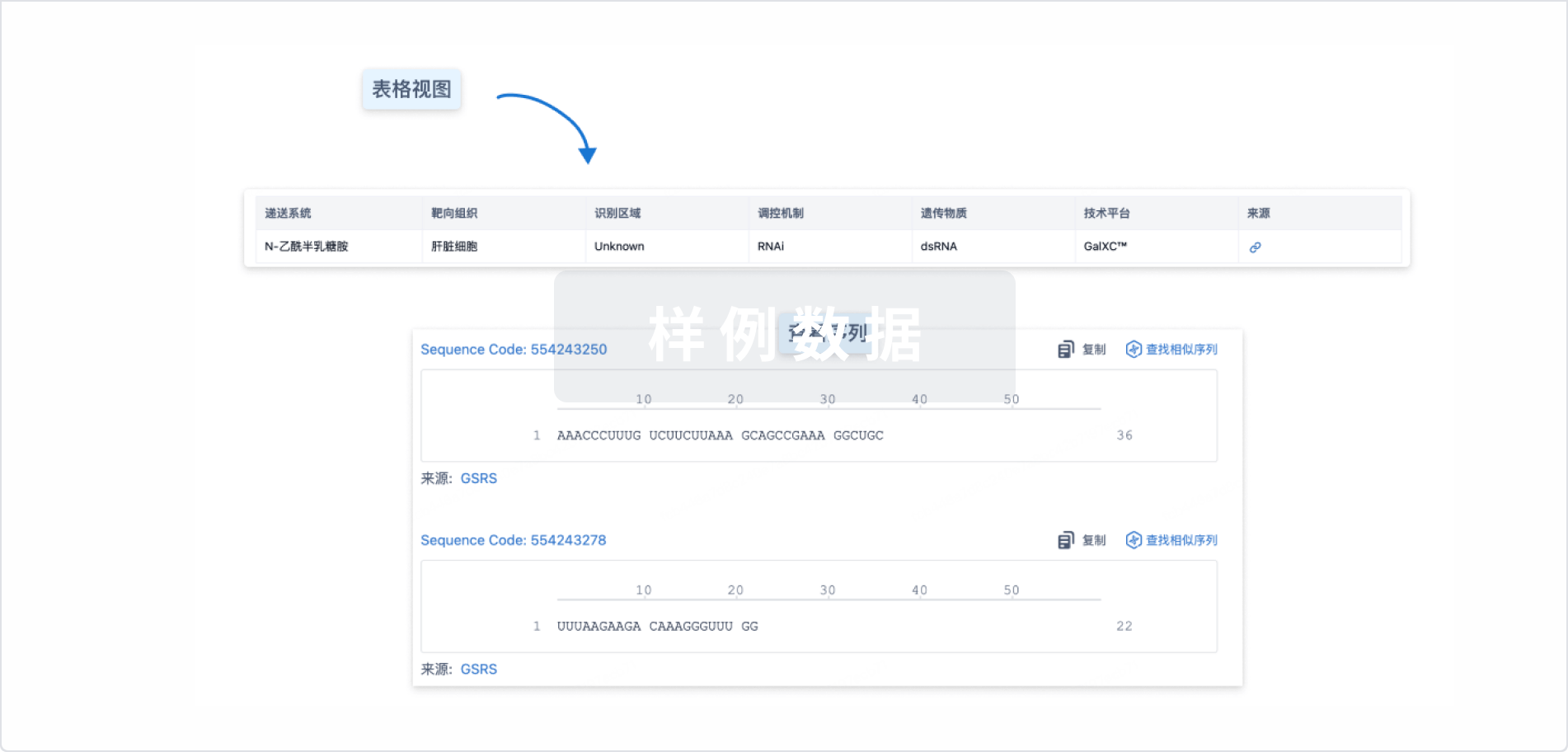
Sequence Code 1277314567

来源: *****
关联
2
项与 IONIS-DNM2-2.5Rx 相关的临床试验NCT04743557
A Phase 1/2, Multicenter, Open-label, Dose-confirmation Trial to Evaluate the Safety and Preliminary Efficacy of DYN101 in Participants 2 to 17 Years of Age With Centronuclear Myopathy Caused by Mutations in MTM1 or DNM2
There are no available treatments aside from supportive care for patients with Centronuclear myopathy (CNM). This trial will assess the safety and tolerability as well as pharmacokinetics (PK), pharmacodynamics (PD) and preliminary efficacy of DYN101 in participants 2 to 17 years of age with CNM caused by mutations in DNM2 or MTM1.The trial will consist of a pre-screening consent, a screening period, a run-in period (if applicable), and a Part 1 of 12 weeks with weekly infusion of DYN101 to evaluate safety and tolerability as well as PK, PD and preliminary efficacy. The dose level may need adjustment based on the Part 1 results of the current study and available data from the Unite-CNM study (DYN101-C101, NCT04033159). If a dose adjustment is needed, Part 2 will be conducted in the same participants and the newly selected dose level will be used to assess whether efficacy is seen after an additional 12 weeks of treatment. As this trial is investigational, there is no defined, expected benefit for subjects who participate in this trial except a better knowledge of their disease.
开始日期2024-01-01 |
申办/合作机构 |
NCT04033159
A Phase 1/2 Trial on the Safety, Tolerability, Pharmacokinetics, Pharmacodynamics and Exploratory Efficacy of DYN101 in Patients ≥ 16 Years of Age With Centronuclear Myopathies Caused by Mutations in DNM2 or MTM1.
There are no available treatments aside from supportive care for patients with Centronuclear myopathy (CNM). This trial will assess the safety, tolerability, pharmacokinetics (PK) and pharmacodynamics (PD)/preliminary efficacy of a new medicine called DYN101 in patients ≥ 16 years of age with CNM caused by mutations in Dynamin2 (DNM2) or Myotubularin1 (MTM1).
The trial will consist of a consent, a screening period, a run-in period (if applicable), a Single dose treatment part (SAD) with 4 weeks of follow-up after the drug administration and a washout period of at least 12 weeks (followed by follow-up phone calls), a Multiple dose treatment part (MAD) of 12 weeks of weekly dosing, and a Multiple dose extension part of 12 weeks. All subjects will participate in the SAD, MAD, and MAD extension parts, unless they withdraw. During this time, multiple test will be performed in order to better understand how the drug is distributed and then later removed from the body and whether there any signs of an effect.
As this trial is investigational, there is no defined, expected benefit for subjects who participate in this trial except a better knowledge of their disease.
The trial will consist of a consent, a screening period, a run-in period (if applicable), a Single dose treatment part (SAD) with 4 weeks of follow-up after the drug administration and a washout period of at least 12 weeks (followed by follow-up phone calls), a Multiple dose treatment part (MAD) of 12 weeks of weekly dosing, and a Multiple dose extension part of 12 weeks. All subjects will participate in the SAD, MAD, and MAD extension parts, unless they withdraw. During this time, multiple test will be performed in order to better understand how the drug is distributed and then later removed from the body and whether there any signs of an effect.
As this trial is investigational, there is no defined, expected benefit for subjects who participate in this trial except a better knowledge of their disease.
开始日期2020-01-09 |
申办/合作机构 |
100 项与 IONIS-DNM2-2.5Rx 相关的临床结果
登录后查看更多信息
100 项与 IONIS-DNM2-2.5Rx 相关的转化医学
登录后查看更多信息
100 项与 IONIS-DNM2-2.5Rx 相关的专利(医药)
登录后查看更多信息
7
项与 IONIS-DNM2-2.5Rx 相关的新闻(医药)2023-03-02
A new formation of Flamingo Therapeutics has taken flight after a merger with Dynacure.
Flamingo Therapeutics is standing on one leg no more, as Dynacure folds into the biotech in a merger agreement that will see the combined company work on RNA-targeted oncology therapies.
The combined company, which will operate as Flamingo Therapeutics, left the lagoon today with Dynacure CEO Stéphane van Rooijen, M.D., leading the flamboyance and Flamingo CEO Michael Garrett at his wing as chief operating officer. Financial details were not disclosed.
Van Rooijen said the biotech ecosystem is currently experiencing “stormy weather,” but said the merger has been in the works since June of last year.
“It's a people business, and we—the management teams—get along,” he told Fierce Biotech in an interview. “The board members get along; we have common investors.”
Proof in point, van Rooijen was joined on the call by COO Garrett, who added that combining the two biotechs’ financial and clinical resources will ideally help get therapies to patients more quickly.
The merged company will focus on launching Flamingo’s danvatirsen in a phase 2 trial with Keytruda in patients with head and neck squamous cell carcinoma (HNSCC). Flamingo obtained rights to test the antisense oligonucleotide as a STAT-3 inhibitor from Ionis in 2021. The trial will compare the combination of danvatirsen and Keytruda against Keytruda alone as a first-line therapy for patients with recurrent or metastatic HNSCC who have tumors with a PD-L1 expression.
Though Flamingo has one preclinical asset called FTX-001—a long non-coding RNA program for solid tumors—discovery work is not the biotech’s focus, CEO van Rooijen explained. Instead, Flamingo is centered on clinical trial execution and its phase 2 study so it can make a significant difference in the future, he said.
The international deal will see the company wade across the pond with a new headquarters set in Belgium, where lab facilities are also located. The biotech will also have offices in France and Philadelphia.
Both biotechs had alliances with Ionis, which will be no different for the new iteration of Flamingo. Ionis’ CEO and President Brett Monia, Ph.D., has joined Flamingo’s board of directors, which will be chaired by Chris Mirabelli, Ph.D., chair of Leap Therapeutics.
As for funding, Flamingo’s current investors Kurma Partners and PMV have put down a further investment in the merged company. When asked about further funding plans, van Rooijen said Flamingo “will not sit back,” and intends to do more fundraising, though he said the company has enough money right now to run the phase 2 trial. When asked about any IPO plans, the CEO expressed caution, citing the need for data from the danvatirsen study first.
“I think it's a bit too early to speak about an IPO. And I don't know if the markets are open for that,” the CEO explained. “But I like the idea—the ambition.”
Dynacure’s most recent fundraise was a $55 million series C in April 2020. The biotech had planned to debut on the market in 2021 but ultimately withdrew its $100 million IPO proposal.
Then, in July of last year, Dynacure called it quits for its lead asset, DYN101, which Ionis had out-licensed to the company back in 2017. The drug was designed to treat rare genetic disorders, specifically myotubular and centronuclear myopathies.

IPO临床2期寡核苷酸高管变更
2022-07-08
Dynacure has ended its Unite-CNM Phase I/II trial on DYN101, an investigational therapy for the treatment of myotubular and centronuclear myopathies (CNM).
In a letter addressed to patients, the company said it is "unable to continue dosing at the low study dose or increase to a dose level at which we would expect to see clinical efficacy."
Dynacure noted that while the first cohort of trial participants, who received a single dose of DYN101, tolerated the drug well, those from the second cohort, who received a higher quantity at the lowest level possible to determine efficacy, experienced elevated liver enzyme levels. The researchers tried to adjust the dose based on the patients' actual to ideal body weight, but all six patients still showed elevated liver enzymes. Five out of the six also reported having low platelet counts.
After reviewing the study with an independent data monitoring committee, it was decided that dosing should be paused. The patients who had already taken the investigational drug continued with their follow-up visits and did not demonstrate any exposure to further risk.
"This is not the news we ever wanted to share, as we know how great your needs are for safe and effective treatments. As a company and as individuals, we are overwhelmed with disappointment," Chris Freitag, M.D., the chief medical officer of Dynacure, said in the letter.
"Like all clinical research efforts into CNM, the learnings from Unite-CNM are hard-earned by these study participants and investigators. They will add to the world's growing understanding of CNM. As we continue to review the findings of Unite-CNM, we will stay connected with study investigators, researchers, and patient advocacy organizations around the world. We will fulfill our obligation of making study results public," Freitag added.
Dynacure had a promising journey prior to this unfortunate news. In June 2021, the U.S. Food and Drug Administration accepted its Investigational New Drug application for DYN101 due to positive initial results from its UNITE-CNM study. In January, the FDA granted it a Fast Track Designation.
"Dynacure is continuing to investigate new approaches to address CNM, but it is too early to comment on any efforts. We remain very grateful for the heroic efforts of the patient who participated in this study, and their families and caregivers who supported these difficult endeavors," the letter concluded.
CNMs are rare, life-threatening disorders that affect skeletal muscles from birth and affect around 4,000 to 5,000 patients in Australia, the European Union, Japan and the U.S. People diagnosed with CNM start experiencing muscle weakness from birth to early adulthood, and many patients die within the first 18 months of life. Those who survive longer usually require intense and uninterrupted medical assistance. Current interventions are merely supportive and not curative.
快速通道
2022-01-07
Biopharma companies are building momentum to carry them through the bulk of 2022. BioSpace takes a look at some of the recent announcements.
Biopharma companies are building momentum to carry them through the bulk of 2022. Companies are providing pipeline updates and forecasts that will position them for potential success. BioSpace takes a look at some of the recent announcements.
Dynacure Snags Fast Track Designation
Pennsylvania-based
Dynacure
received Fast Track designation from the U.S. Food and Drug Administration (FDA) for lead candidate
DYN101
, a potential treatment for Myotubular and Centronuclear Myopathies (CNM), a group of rare genetic diseases. A Fast Track designation provides expedited development and review of drugs for indications with significant unmet medical needs.
CNM is driven by mutations in multiple genes, including MTM1, DNM2, and BIN. Dynacure said its researchers had discovered a link between an increase in DNM2 protein and the direct cause of the disease.
Dynacure is currently assessing DYN101 in the Phase I/II UNITE-CNM study. DYN101 is being studied in that trial as a treatment for the two most common forms of CNM—X-linked myotubular myopathy (XLMTM) and autosomal dominant CNM (ADCNM). The company anticipates initial data from the ascending dose study in the second half of this year.
Freeline Therapeutics Receives Greenlight for Gaucher Type 1 Gene Therapy Trial
London-based
Freeline Therapeutics
is expected to initiate a Phase I study of experimental gene therapy to treat Gaucher disease Type 1. The
FDA cleared
the company’s Investigational New Drug (IND) application for FLT201, an AAV capsid (AAVS3) that contains an expression cassette, which encodes for a novel glucocerebrosidase variant (GCasevar85) under the control of a liver-specific promoter.
Michael Parini, chief executive officer of Freeline, said FLT201 is the first AAV-mediated gene therapy for Gaucher disease Type 1 to enter the clinic. Prior to FDA approval of the IND, Freeline had also received clearance to begin a Phase I/II dose-finding study of FLT201 in Europe. Later this year, the first patients are expected to be dosed in that study. Initial safety and biomarker data from the first cohort is expected in the third quarter.
Freeline’s preclinical proof-of-concept studies suggest that FLT201 has the potential to reach challenging to treat tissues that are not sufficiently addressed by current standard-of-care enzyme replacement therapy. The company believes that FLT201 can be a transformative product for Gaucher Type 1.
Neurocrine to Focus on Expansion of Ingrezzia, Other Clinical Programs
As San Diego-based
Neurocrine
looks ahead into 2022, the company said its
priorities are squarely focused on Ingrezzia
(valbenazine), which the FDA first approved in 2017 for the treatment of tardive dyskinesia (TD). The company assesses its asset in multiple indications, including Chorea in Huntington’s disease. Neurocrine anticipates a supplemental New Drug Application will be filed for this indication in the second half of 2022.
Neurocrine CEO Kevin Gorman said the company is well-positioned to drive the growth of Ingrezzia in TD and other indications, which will then support the development of its 13 other clinical programs in mid-to-late stage studies.
“We exited 2021 helping more patients with tardive dyskinesia than ever before. Furthermore, we now have 13 clinical programs in mid-to-late-stage studies which will generate important data readouts over the next two years,” Gorman said in a statement.
Mersana Therapeutics Unveils Big Plans for 2022
Cambridge, Mass.-based
Mersana
is also looking forward to 2022. This morning, the company announced
multiple goals and milestones
for the coming 12 months. Chief among those priorities is its plan to build upifitamab rilsodotin (UpRi) as a foundational medicine to treat ovarian cancer. UpRi is a first-in-class dolaflexin ADC that targets NaPi2b. Mersana enrolls patients in the UPLIFT clinical study, a registrational trial in platinum-resistant ovarian cancer. Enrollment is expected to be completed by the third quarter of this year.
Additionally, the company is conducting the Phase III UP-NEXT study with UpRi in platinum-sensitive recurrent ovarian cancer. It also is running UPGRADE, a Phase I/II combination study in platinum-sensitive ovarian cancer. Interim data is expected from this study in the second half of 2022.
In addition to UpRi, Mersana also assesses other oncology drugs, including XMT-1592, a first Dolasynthen ADC that targets NaPi2b and XMT-1660, a first-in-class Dolasynthen ADC targeting B7-H4.
The company also plans to announce more information about two development candidates, XMT-2068 and XMT-2175, later this year. Both are STING-agonist ADCs targeting tumor-associated antigens.
临床3期上市批准快速通道临床1期临床申请
100 项与 IONIS-DNM2-2.5Rx 相关的药物交易
登录后查看更多信息
研发状态
10 条进展最快的记录, 后查看更多信息
登录
| 适应症 | 最高研发状态 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|---|
| 中央核肌病 | 临床2期 | 比利时 | 2020-01-09 | |
| 中央核肌病 | 临床2期 | 丹麦 | 2020-01-09 | |
| 中央核肌病 | 临床2期 | 法国 | 2020-01-09 | |
| 中央核肌病 | 临床2期 | 德国 | 2020-01-09 | |
| 中央核肌病 | 临床2期 | 荷兰 | 2020-01-09 | |
| 中央核肌病 | 临床2期 | 英国 | 2020-01-09 |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
No Data | |||||||
登录后查看更多信息
转化医学
使用我们的转化医学数据加速您的研究。
登录
或

药物交易
使用我们的药物交易数据加速您的研究。
登录
或

核心专利
使用我们的核心专利数据促进您的研究。
登录
或

临床分析
紧跟全球注册中心的最新临床试验。
登录
或

批准
利用最新的监管批准信息加速您的研究。
登录
或

特殊审评
只需点击几下即可了解关键药物信息。
登录
或

生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用


