预约演示
更新于:2026-02-28
PYC-003
更新于:2026-02-28
概要
基本信息
药物类型 ASO、多肽偶联药物(PDC) |
别名 PYC003 |
靶点 |
作用方式 调节剂 |
作用机制 PKD1 modulators(polycystin 1, transient receptor potential channel interacting modulators)、RNA干扰 |
非在研适应症- |
非在研机构- |
权益机构- |
最高研发阶段临床1期 |
首次获批日期- |
最高研发阶段(中国)- |
特殊审评- |
登录后查看时间轴
结构/序列
使用我们的ADC技术数据为新药研发加速。
登录
或

关联
1
项与 PYC-003 相关的临床试验NCT06714006
A Phase 1 Study to Evaluate the Safety, Tolerability, and Pharmacokinetics of Intravenously Administered PYC-003, a Peptide-phosphorodiamidate Morpholino Oligonucleotide Conjugate, in Healthy Adult Participants and Adult Participants With Confirmed PKD1 Mutation-associated Autosomal Dominant Polycystic Kidney Disease
This is a Phase 1, First-in-Human study to evaluate the safety, tolerability, pharmacokinetics (PK), pharmacodynamics (PD) and immunogenicity of PYC-003 in healthy adult participants and adult participants with confirmed PKD1 mutation-associated Autosomal Dominant Polycystic Kidney Disease (ADPKD) There are 2 parts in this study, i.e. Part A and Part B.
开始日期2025-04-07 |
申办/合作机构 |
100 项与 PYC-003 相关的临床结果
登录后查看更多信息
100 项与 PYC-003 相关的转化医学
登录后查看更多信息
100 项与 PYC-003 相关的专利(医药)
登录后查看更多信息
2
项与 PYC-003 相关的新闻(医药)2025-06-23
PYC has developed a new drug candidate for the >5 million people worldwide[1] with Polycystic Kidney Disease (PKD)
This drug candidate has
demonstrated efficacy in human models derived from the kidneys of patients with end-stage renal failure due to PKD
[2]
PKD is a life-changing disease affecting 1 in every 1,000 people[3]
Half of the patient population with PKD will require a kidney transplant by the age of 60 due to the absence of impactful treatment options in this disease[4]
PKD represents an addressable market of
>US$10 billion p.a. and is an area of major commercial interest for the drug development industry[5]
Pre-clinical models suggest that addressing the underlying cause of PKD (the way that PYC's drug candidate works) could
arrest the course of the disease in humans, enabling damaged kidneys to
regenerate and
restore function[6]
PYC plans to accelerate this drug candidate into human trials in ~12 months – pursuing a potential high-velocity path through clinical trials to market[7]
Investor Webinar Friday 17 November
PYC will host an investor call at 9am AWST/12pm AEDST on Friday 17 November to discuss these results – investors can register for the call here:
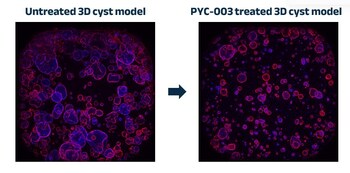
PERTH, Australia and SAN FRANCISCO, Nov. 13, 2023 /PRNewswire/ -- PYC Therapeutics today announces the results of a study conducted in human 3-dimensional models derived from patients with end-stage renal failure due to Autosomal Dominant Polycystic Kidney Disease (PKD). The results demonstrate that an investigational drug candidate designed by PYC (known as PYC-003) to address this disease at its root cause is effective. These 3D patient-derived cyst models represent the 'gold-standard' pre-clinical assay for evaluating drug candidates in this indication[8].
Continue Reading
Figure 1
Figure 2
Figure 3
Figure 4
2025-06-05
关注并星标CPHI制药在线RNA疗法正迅速成为现代医学中最具变革性的治疗策略之一。从反义寡核苷酸(ASO)和小干扰RNA(siRNA)等精准调控基因表达的工具,到mRNA疫苗和肿瘤免疫疗法等前沿应用,RNA技术已在多个疾病领域展现出巨大潜力。近年来,随着递送系统、生物偶联技术和修饰化学的不断进步,RNA疗法的临床管线迅速扩展,涵盖了罕见病、神经系统疾病、代谢性疾病以及肿瘤等多个领域。目前,RNA疗法的临床开发全景涵盖了ASO、siRNA、mRNA、核酸适体(aptamer)等主要技术路径,在不同适应症中布局广泛,关键企业的研发动态频繁,已获批上市的代表性药物不断涌现。本文总结了现有管线和上市的 RNA疗法,将这一新兴领域的开发全景呈现出来。1. ASO类 RNA资产梳理 1.1 I期ASO资产 • Praxis ASO资产 :Elsunersen(PRAX-222) 靶点:SCN2A基因 适应症:由SCN2A基因的功能获得性突变引起的早发性SCN2A发育性和癫痫性脑病。 • PYC Therapeutics ASO资产:VP-001 靶点:PRPF31基因 适应症:由PRPF31基因突变引起的视网膜色素变性11型 ASO资产:PYC-001 靶点:OPA1基因 适应症:由OPA1基因突变引起的常染色体显性视神经萎缩(ADOA) ASO资产:PYC-003 靶点:PKD1基因 适应症:常染色体显性多囊肾病(ADPKD) • Bio-Path Holdings ASO资产:BP1002(Liposomal Grb2 ASO) 适应症:急性髓性白血病(AML)、B细胞淋巴瘤(B-cell lymphoma) 技术特点:使用独特的中性脂质体递送系统(DNAbilize® Platform)递送ASO。1.2 II期ASO资产 • Stoke Therapeutics ASO资产:Zorevunersen(STK-001) 靶点 :SCN1A基因 适应症:Dravet综合征(一种严重癫痫) ASO资产 :STK-002 靶点:OPA1基因 适应症:常染色体显性视神经萎缩(ADOA) • Entrada Therapeutics ASO资产:ENTR-601-44 适应症:Duchenne肌营养不良(DMD) 靶点 :DMD中的Exon 44跳跃 技术特点:使用Entrada独有的分子穿透技术(Endosomal Escape Vehicle, EEV)提高细胞内递送效率。 ASO资产:ENTR-601-45 靶点:DMD基因的外显子45(Exon 45) 适应症:适用于外显子45跳跃的杜氏肌营养不良症(DMD)患者 • Dyne Therapeutics ASO资产:DYNE-251 适应症:Duchenne肌营养不良(DMD),针对DMD中的Exon 51跳跃。 技术特点:使用抗体-寡核苷酸结合平台(FORCE platform)进行肌肉组织特异性递送。 ASO资产 :DYNE-101 适应症:肌强直性营养不良症1型(DM1) 靶点:DMPK基因的异常CTG重复序列 • Ribomic ASO资产:Umedaptanib Pegol(原RBM-007,严格来说是核酸适体aptamer,而不是典型ASO) 靶点 :FGF2(成纤维细胞生长因子2)。 适应症:湿性年龄相关性黄斑变性(wAMD),软骨发育不全症 • Wave Life Sciences ASO资产: WVE-N531:DMD(杜氏肌营养不良症)治疗,靶向Exon 53跳跃 WVE-003:亨廷顿舞蹈症(Huntington's Disease, HD),靶向HTT基因突变 • PepGen ASO资产:PGN-EDO51 适应症:Duchenne肌营养不良(DMD) 技术特点:结合增强型细胞穿透肽(Enhanced Delivery Oligonucleotide, EDO)技术,提高ASO进入肌肉细胞的效率。 ASO资产 :PGN-EDODM1 靶点:DMPK基因的异常CUG重复序列 适应症:肌强直性营养不良症1型(DM1) • 罗氏 ASO资产 :Tominersen(RG6042) 靶点:Huntingtin(HTT)基因 适应症:亨廷顿病(Huntington's Disease, HD) ASO资产 :IONIS-FB-LRx(RO7434656 / RG6299) 靶点:补体因子B(Factor B) 适应症:IgA肾病(IgAN) • Ultragenyx ASO资产 :GTX-102 靶点:UBE3A-AS(UBE3A反义转录本) 适应症:天使综合征(Angelman Syndrome)1.3 ASO类3期资产 • Oncotelic Therapeutics ASO资产:OT-101(也叫Trabedersen) 靶点:转化生长因子β2(TGF-β2) 适应症:胶质母细胞瘤(GBM)、胰腺癌、恶性黑色素瘤 机制:抑制TGF-β2,有望减轻肿瘤免疫抑制,增强抗肿瘤免疫反应 • 阿斯利康 ASO资产:ION449(亦称AZD8233),与Ionis合作开发的ASO疗法 靶点:PCSK9(一种影响低密度脂蛋白胆固醇LDL-C水平的蛋白质) 适应症:高胆固醇血症(hypercholesterolemia) 备注:主要竞争对手包括小分子抑制剂和PCSK9单抗(如Repatha、Praluent) • GSK ASO资产:bepirovirsen 靶点:乙型肝炎病毒(HBV)的前基因组RNA和mRNA 适应症:慢性乙型肝炎(chronic hepatitis B, CHB),通过抑制HBV基因表达,帮助病毒清除或实现功能性治愈(functional cure)。 • 诺华 ASO资产:Pelacarsen 靶点:LPA基因(编码脂蛋白(a)) 作用机制:Pelacarsen通过抑制LPA基因的mRNA,降低血液中脂蛋白(a)(Lp(a))水平,从而降低心血管疾病风险。Lp(a)是目前被认为的重要但尚无针对性治疗手段的心血管风险因子。 适应症:主要用于治疗动脉粥样硬化性心血管疾病高危患者中,血脂异常(特别是Lp(a)升高)的情况。 临床阶段:正在进行III期临床试验(Lp(a)HORIZON)。Pelacarsen可能成为首个专门针对Lp(a)升高治疗获批的药物。1.4 ASO类上市药物 • 渤健 Spinraza(nusinersen) 靶点:SMN2前体mRNA(促进功能性SMN蛋白表达) 适应症:脊髓性肌萎缩症(SMA) 获批时间:2016年12月(美国FDA批准) 备注:全球首个获批的SMA治疗药物。由Ionis开发,Biogen负责商业化。 • Sarepta Therapeutics Exondys 51(eteplirsen) 靶点:DMD基因的Exon 51 适应症:Duchenne肌营养不良(DMD) 获批时间:2016年9月(FDA加速批准) Vyondys 53(golodirsen) 靶点:DMD基因的Exon 53 适应症:DMD 获批时间:2019年12月 Amondys 45(casimersen) 靶点:DMD基因的Exon 45 适应症:DMD 获批时间:2021年2月(FDA加速批准) • Ionis Pharmaceuticals Tegsedi(inotersen) 靶点:TTR(转甲状腺素蛋白) 适应症:遗传性转甲状腺素蛋白淀粉样变性多发性神经病(hATTR-PN) 获批时间 :2018年10月(FDA,EMA批准) Waylivra(volanesorsen) 靶点:APOC3(载脂蛋白C-III) 适应症:家族性乳糜微粒血症综合征(FCS) 获批时间:2019年5月(欧洲EMA批准;未获美国FDA批准) • NS Pharma Viltepso(viltolarsen) 靶点:DMD基因的Exon 53 适应症:Duchenne肌营养不良(DMD) 获批时间:2020年8月(FDA加速批准)2.RNAi类 RNA资产2.1 I期RNAi资产 • Aligos Therapeutics RNAi资产:ALG-125755 靶点:乙型肝炎病毒(HBV)RNA 适应症:慢性乙型肝炎(CHB) • Regulus Therapeutics RNAi资产:RGLS8429 靶点:microRNA-17(miR-17家族) 适应症:常染色体显性多囊肾病(ADPKD) • Phio Pharmaceuticals RNAi资产:PH-762 靶点:程序性死亡受体1(PD-1) 适应症:癌症免疫疗法(通过下调T细胞表面PD-1,增强免疫杀伤) 技术特点:采用其自有的INTASYL✓平台,优化了siRNA的稳定性和局部递送。 • TransCode Therapeutics RNAi资产:VIR-2218 TTX-MC138 靶点:microRNA-10b(miR-10b) 适应症:针对转移性实体肿瘤(如乳腺癌、胰腺癌、脑肿瘤等) 技术特点:使用纳米颗粒系统高效递送siRNA药物,特别适合针对深部转移灶。2.2 II期RNAi资产 • Arbutus Biopharma RNAi资产:AB-729 靶点:乙型肝炎病毒(HBV)表面抗原(HBsAg) 适应症:慢性乙型肝炎(CHB) 备注:AB-729是Arbutus当前最重要的核心资产之一。 • OliX Pharmaceuticals RNAi资产:OLX703A 靶点:HBV病毒相关RNA 适应症:慢性乙型肝炎(CHB) 机制:抑制HBV病毒蛋白表达 • Silence Therapeutics RNAi资产:SLN360 靶点:LPA基因(编码脂蛋白Lp(a)) 适应症:高脂蛋白(a)血症(Lp(a)过高相关的心血管疾病) 机制:通过降低血液中Lp(a)水平,减少动脉粥样硬化风险。 备注:SLN360是Silence在心血管RNA疗法领域的主力项目。 • Sirnaomics RNAi资产:STP705 靶点:双靶点siRNA药物,靶向TGF-β1和COX-2。 适应症:瘢痕治疗(皮肤纤维化)、非黑色素瘤皮肤癌(如基底细胞癌、鳞状细胞癌) 机制:抑制纤维化、降低肿瘤微环境免疫抑制。 备注:Sirnaomics的特点是双靶点siRNA联合递送。 • GSK RNAi资产:GSK4532990 靶点:靶向HSD17B13基因 适应症:代谢功能障碍相关脂肪性肝炎(MASH) • 礼来 RNAi项目:LY3561774 靶点:SERPINA1基因 适应症:α1-抗胰蛋白酶缺乏症(AATD) 机制 :降低异常α1-抗胰蛋白酶(AAT)积聚 • VIR Biotechnology RNAi项目:VIR-2218 靶点 : HBV RNA 适应症:慢性乙型肝炎(CHB)。 机制:抑制HBV表面抗原表达 • 罗氏 RNAi资产:Zilebesiran 靶点 :肝脏表达的血管紧张素原(AGT)基因。 适应症:高血压,特别是高心血管风险患者。2.3 III期RNAi资产 • Arrowhead Pharmaceuticals RNAi资产:ARO-APOC3 靶点 :APOC3(载脂蛋白C-III), 适应症:高甘油三酯血症和罕见脂代谢异常 • 武田 RNAi资产:Fazirsiran 靶点:SERPINA1(编码α1-抗胰蛋白酶) 适应症:α1-抗胰蛋白酶缺乏症(AATD)引起的肝病 • 安进 RNAi资产:Olpasiran 靶点:LPA基因(脂蛋白(a)) 适应症:高脂蛋白(a)血症及其引发的心血管疾病风险 备注:这是一款非常重磅的RNAi项目,对心血管领域意义重大。 • Avidity Biosciences RNAi资产:AOC 1001 靶点:DMPK基因(导致肌强直性营养不良症1型DM1) 适应症:肌强直性营养不良症1型(DM1) 技术特点:AOC(Antibody Oligonucleotide Conjugate,抗体-寡核苷酸偶联体),用于肌肉靶向递送siRNA。 • 赛诺菲 RNAi资产:Fitusiran 靶点:AT(抗凝血酶) 适应症:血友病A或B患者的出血预防 • VIR Biotechnology RNAi资产:VIR-2218 靶点:HBV RNA 适应症:慢性乙型肝炎(CHB)2.4 上市RNAi资产 • Alnylam Pharmaceuticals Alnylam是全球RNAi疗法先锋,拥有最多已上市RNAi药物。 Onpattro(Patisiran) 靶点:TTR 适应症:遗传性转甲状腺素蛋白淀粉样变性引起的多发性神经病(hATTR-PN) 批准情况:2018年FDA/EMA批准 Givlaari(Givosiran) 靶点:ALAS1 适应症:急性肝卟啉症(AHP) 批准情况:2019年FDA/EMA批准 Oxlumo(Lumasiran) 靶点:HAO1 适应症:原发性高草酸尿症1型(PH1) 批准情况:2020年FDA/EMA批准 Amvuttra(Vutrisiran) 靶点:TTR 适应症:遗传性转甲状腺素蛋白淀粉样变性引起的多发性神经病(hATTR-PN)(长效皮下注射版) 批准情况:2022年FDA批准 • 诺华 RNAi资产:Leqvio(Inclisiran) 靶点:PCSK9 适应症:高胆固醇血症,动脉粥样硬化性心血管疾病风险降低 批准情况:2020年欧洲EMA批准;2021年底美国FDA批准 • 诺和诺德 RNAi资产:Rivfloza (nedosiran) 靶点:肝脏中乳酸脱氢酶(LDH)mRNA 适应症:治疗9岁及以上、肾功能相对良好的原发性高草酸尿症1型(PH1)患者。 作用机制:抑制LDH蛋白表达,减少草酸过度生成,缓解PH1疾病过程,减少肾脏损害风险。 批准情况:2023年9月29日FDA正式批准Rivfloza上市。成为继Alnylam的Oxlumo(Lumasiran)之后,全球第二款获批用于PH1治疗的RNAi药物。3.mRNA类 RNA药物资产3.1 1期mRNA资产 • 赛诺菲 SP0237: 季节性流感 mRNA 疫苗 适应症:针对 A/H3N2 毒株的季节性流感。 SP0256 – RSV mRNA 疫苗(老年人群) 适应症:用于预防老年人群中的呼吸道合胞病毒(RSV)感染。 SP0268 – mRNA 痤疮疫苗 适应症:针对痤疮(Acne)的 mRNA 疫苗。 SP0291 – 多价呼吸道病毒 mRNA 疫苗 适应症:针对 RSV、人类偏肺病毒(hMPV)和副流感病毒 3 型(PIV3)的组合疫苗,主要用于老年人群。 沙眼衣原体(Chlamydia)mRNA 疫苗候选 适应症:预防沙眼衣原体感染,主要针对 18 至 29 岁的成人。 • Vertex mRNA资产:VX-522:用于囊性纤维化(CF)的mRNA疗法 靶点:CFTR基因(囊性纤维化跨膜电导调节因子)。 适应症:无法通过CFTR调节剂治疗的CF(囊性纤维化)患者,尤其是那些由于CFTR基因突变导致CFTR蛋白缺失的患者。 作用机制:VX-522是一种mRNA疗法,通过脂质纳米颗粒(LNP)递送CFTR mRNA至肺部细胞,促使其合成功能性CFTR蛋白,从而恢复氯离子通道的功能。3.2 II期mRNA资产 • Gritstone Bio GRANITE(GRT-C901/GRT-R902) 个体化肿瘤新抗原疫苗,采用腺病毒(ChAd68)和自扩增mRNA(samRNA)组成的异源prime-boost策略。 适应症:用于治疗转移性微卫星稳定型结直肠癌(MSS-CRC)。 CORAL 基于自扩增mRNA(samRNA)的COVID-19疫苗,诱导广泛且持久的中和抗体和T细胞免疫反应。 • CureVac/GSK 季节性流感mRNA疫苗候选 该疫苗候选基于CureVac的第二代mRNA平台,针对流感病毒株,包括A型和B型毒株。 COVID-19 mRNA疫苗候选CV0601和CV0701 CV0601为单价疫苗,编码Omicron BA.4/5变异株的刺突蛋白;CV0701为二价疫苗,编码Omicron BA.4/5变异株和原始SARS-CoV-2毒株的刺突蛋白。3.3 III期mRNA资产 • 第一三共 DS-5670a(原始毒株单价疫苗) 适应症:Covid-19疫苗,作为加强针用于已完成初始疫苗接种的成人和老年人。 DS-5670a/b(原始毒株与Omicron BA.4/5二价疫苗) 适应症:Covid-19疫苗,作为加强针用于12岁及以上已完成初始疫苗接种的人群。 DS-5670d(Omicron XBB.1.5单价疫苗) 适应症:Covid-19疫苗,作为季节性加强针用于12岁及以上人群,包括未接种疫苗者。 • 默沙东 mRNA资产:V940(mRNA-4157) 类型:肿瘤疫苗(个体化mRNA疫苗) 适应症:手术切除后的高风险黑色素瘤患者辅助治疗 靶点:个体化新抗原(根据患者肿瘤突变特异设计)3.4上市mRNA资产 • Arcturus Therapeutics mRNA资产:KOSTAIVE(ARCT-154 / Zapomeran) 类型:自扩增mRNA(sa-mRNA)疫苗 适应症:用于预防COVID-19,适用于18岁及以上人群 靶点:SARS-CoV-2病毒刺突蛋白(Spike protein) • BioNTech/辉瑞 Comirnaty 类型:mRNA疫苗 适应症:用于预防COVID-19,适用于6个月及以上人群 靶点:SARS-CoV-2病毒刺突蛋白(Spike protein) 获批时间:✓2020年12月获得FDA紧急使用授权,2021年8月获得美国FDA完全批准 • 莫德纳 Spikevax 类型:mRNA疫苗 适应症:用于预防COVID-19,适用于6个月及以上人群 靶点:SARS-CoV-2病毒刺突蛋白(Spike protein) 获批时间:2020年12月18日,美国FDA授予紧急使用授权;2022年1月31日,获得FDA完全批准Ref. TD Cowen. Pharmaceuticals’ websitesEND【企业推荐】来源:CPHI制药在线声明:本文仅代表作者观点,并不代表制药在线立场。本网站内容仅出于传递更多信息之目的。如需转载,请务必注明文章来源和作者。投稿邮箱:Kelly.Xiao@imsinoexpo.com▼更多制药资讯,请关注CPHI制药在线▼点击阅读原文,进入智药研习社~
临床2期寡核苷酸上市批准信使RNA临床1期
100 项与 PYC-003 相关的药物交易
登录后查看更多信息
研发状态
10 条进展最快的记录, 后查看更多信息
登录
| 适应症 | 最高研发状态 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|---|
| 常染色体显性多囊肾病 | 临床1期 | 澳大利亚 | 2025-04-07 | |
| 常染色体显性多囊肾病 | 临床1期 | 新西兰 | 2025-04-07 | |
| Phelan-McDermid 综合征 | 临床前 | 澳大利亚 | 2022-10-20 |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
No Data | |||||||
登录后查看更多信息
转化医学
使用我们的转化医学数据加速您的研究。
登录
或

药物交易
使用我们的药物交易数据加速您的研究。
登录
或

核心专利
使用我们的核心专利数据促进您的研究。
登录
或

临床分析
紧跟全球注册中心的最新临床试验。
登录
或

批准
利用最新的监管批准信息加速您的研究。
登录
或

特殊审评
只需点击几下即可了解关键药物信息。
登录
或

生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用
