预约演示
更新于:2026-01-17
Butalbital
布他比妥
更新于:2026-01-17
概要
基本信息
药物类型 小分子化药 |
别名 5-(2-methylpropyl)-5-prop-2-enyl-1,3-diazinane-2,4,6-trione、5-allyl-5-(2'-methyl-n-propyl) barbituric acid、5-allyl-5-(2-methylpropyl)barbituric acid + [13] |
作用方式 激动剂 |
作用机制 GABAA receptor激动剂(γ-氨基丁酸 A 受体激动剂) |
治疗领域- |
在研适应症- |
非在研适应症- |
原研机构- |
在研机构- |
非在研机构- |
权益机构- |
最高研发阶段- |
首次获批日期- |
最高研发阶段(中国)- |
特殊审评- |
结构/序列
分子式C11H16N2O3 |
InChIKeyUZVHFVZFNXBMQJ-UHFFFAOYSA-N |
CAS号77-26-9 |
关联
100 项与 布他比妥 相关的临床结果
登录后查看更多信息
100 项与 布他比妥 相关的转化医学
登录后查看更多信息
100 项与 布他比妥 相关的专利(医药)
登录后查看更多信息
517
项与 布他比妥 相关的文献(医药)2025-04-01·AMERICAN FAMILY PHYSICIAN
Acute Migraine Headache: Treatment Strategies.
Review
作者: Lehmann, Delaney N ; Watson, James C ; Wiley, Anna T
Migraine is a primary headache disorder characterized by recurrent disabling attacks. Pharmacologic treatment of acute migraine episodes should be individualized based on route of administration, cost, contraindications, and adverse effects. Stratifying treatment based on migraine severity may result in more rapid resolution of symptoms and return of function. Simple analgesics, such as acetaminophen and nonsteroidal anti-inflammatory drugs, are first-line treatments for mild to moderate migraine episodes, and triptans are first-line therapy for moderate to severe attacks. Antiemetics and ergot alkaloids are recommended as second-line agents and in cases of refractory migraine. Gepants and ditans are promising newer agents that are supported by quality evidence for second-line use. Unlike triptans and ergot alkaloids, gepants and ditans do not have vascular contraindications. The use of these medications is largely limited by cost, although the adverse effects of ditans also may limit their use. Opioids and butalbital-containing medications are not recommended for the treatment of migraine unless other options have been ineffective. There is insufficient evidence to recommend nonpharmacologic therapies, such as neuromodulatory devices, acupuncture, and greater occipital nerve blocks, but these therapies may be appropriate for select patients.
2024-12-01·Health Science Reports
Association Between Spinal Manipulation, Butalbital Prescription, and Medication Overuse Headache in Adults With Tension‐Type Headache: Retrospective Cohort Study
Article
作者: Pratheek S. Makineni ; Lindsay H. Morris ; Timothy J. Williamson ; Robert J. Trager
ABSTRACT:
Background and Aims:
Butalbital is an acute headache medication commonly prescribed for tension‐type headache (TTH), although discouraged by guidelines due to a risk of medication overuse headache (MOH). Considering spinal manipulative therapy (SMT) may reduce TTH frequency and intensity, we hypothesized adults with TTH receiving chiropractic SMT would be less likely to receive a butalbital prescription over 2 years versus matched controls not receiving SMT. We secondarily compared likelihood of MOH between cohorts.
Methods:
We searched a United States medical records database of patients attending academic medical centers for adults with TTH, from 2013 to 2024, excluding those diagnosed with other headaches and seen in inpatient/emergency settings. We divided patients into two cohorts: (1) SMT and (2) non‐SMT, using propensity matching to control for demographics and other variables associated with likelihood of butalbital prescription and MOH.
Results:
Three thousand one hundred and sixteen patients remained per cohort after matching. The incidence of butalbital prescription was lower in the SMT cohort compared to the non‐SMT cohort (SMT: 1.7%; non‐SMT: 3.8%), yielding an RR (95% CI) of 0.46 (0.33–0.63; p < 0.001). The incidence of MOH was lower in the SMT cohort versus non‐SMT cohort (SMT: 0.5%; non‐SMT: 1.2%), yielding an RR (95% CI) of 0.44 (0.25–0.80; p < 0.001).
Conclusion:
Adults receiving chiropractic SMT had a significantly lower likelihood of butalbital prescription and, tentatively, MOH compared to matched controls not receiving SMT. These findings support current guideline recommendations favoring SMT in TTH care, though future studies should replicate and compare these findings with other nonpharmacologic clinicians and interventions.
2024-11-08·Cureus Journal of Medical Science
Pneumocephalus After Lumbar Epidural Steroid Injection: A Rare Complication With Spontaneous Resolution
Article
作者: Stern, Roger ; Gupta, Ishank ; Hegazy, Yasser ; Ghallab, Muhammad ; Balassiano, Natalie N
Pneumocephalus is a rare but potentially serious complication of spinal procedures, characterized by the presence of intracranial air. This report presents the case of a 40-year-old female who developed pneumocephalus following a lumbar epidural steroid injection. She presented to the emergency department with a persistent headache, blurred vision, and eye pain, which began shortly after the procedure. Computed tomography (CT) scans of the head and lumbar spine revealed several air pockets in the cerebellar cisterns and the left frontal horn, as well as in the epidural and paraspinal regions. Despite the presence of intracranial air, the patient's symptoms gradually improved with conservative management, including bed rest, caffeinated drinks, intravenous fluids, and symptomatic relief with butalbital-acetaminophen-caffeine. A repeat CT scan on day four showed a reduction in air pockets, and by day 10, all air pockets had resolved without the need for neurosurgical intervention. The patient's headaches subsided, though she experienced mild residual vision changes. This case emphasizes the importance of recognizing pneumocephalus as a potential complication of epidural steroid injections and highlights the efficacy of conservative treatment. While most cases of simple pneumocephalus resolve spontaneously, careful monitoring is essential to prevent progression to tension pneumocephalus, a life-threatening condition that requires urgent surgical intervention. Further studies are needed to evaluate the risks and outcomes of different techniques used during epidural procedures.
3
项与 布他比妥 相关的新闻(医药)2025-12-26
一、安眠药中毒的基础认知与急诊识别
1.1 安眠药的分类体系与毒理特点
在急诊工作中,我们经常会遇到因服用过量安眠药而前来就诊的患者。了解安眠药的分类和毒理特点,是正确诊断和治疗的基础。
安眠药的分类方法有很多种,最常用的是根据药物的化学结构和药理作用进行分类。第一代安眠药主要是巴比妥类药物,这类药物早在1864年就被人工合成,但到1903年才发现它具有镇静作用。巴比妥类药物根据作用时间长短分为四类:长效类(作用时间6-8小时)包括巴比妥和苯巴比妥(鲁米那);中效类(作用时间3-6小时)包括戊巴比妥、异戊巴比妥、布他比妥;短效类(作用时间2-3小时)包括司可巴比妥、硫喷妥钠;超短效类如硫喷妥钠等。
第二代安眠药是苯二氮卓类药物,这是上世纪50年代问世的一类新型安眠药。这类药物与GABA受体多位点结合,通过调节神经递质产生镇静、催眠、抗焦虑、抗惊厥和肌肉松弛等多种药理作用。根据半衰期长短,苯二氮卓类药物又分为长效类(如地西泮、氯硝西泮、氟西泮)、中效类(如艾司唑仑、阿普唑仑、劳拉西泮)和短效类(如三唑仑)。
第三代安眠药是非苯二氮卓类药物,也被称为"Z-药物",是上世纪90年代问世的一类新型安眠药。这类药物与GABA受体结合位点比较单一,其催眠作用更为单纯。主要包括唑吡坦(1988年上市)、扎来普隆(1999年上市)和佐匹克隆/右佐匹克隆。唑吡坦15-30分钟起效,作用持续3-6小时;佐匹克隆半衰期5-7小时,对入睡和睡眠维持障碍均有效;右佐匹克隆是佐匹克隆的右旋异构体,疗效更佳,不良反应少。
近年来,又出现了一些新型安眠药。2023年10月1日起,地达西尼被列入第二类精神药品目录,它属于新型苯二氮卓受体激动剂。此外,还有双食欲素受体拮抗剂(如苏沃雷生)、褪黑素受体激动剂等新型安眠药正在临床应用或研发中。
1.2 中毒程度评估与临床表现
安眠药中毒的严重程度与服用药物的种类、剂量、时间以及患者的个体差异密切相关。根据临床表现,安眠药中毒可分为三度:
轻度中毒时,患者主要表现为嗜睡、判断力和定向力障碍、步态不稳、言语不清、眼球震颤,但各种反射正常。患者意识清醒或轻度嗜睡,生命体征基本正常,一般血压、呼吸等都在正常范围内。
中度中毒时,患者进入浅昏迷状态,强刺激可唤醒但不能回答问题,腱反射消失,呼吸浅慢。患者呈深睡状态,言语很难唤醒,但对刺痛可有反应,呼吸浅、心跳减慢,尚有规律,血压仍可正常。
重度中毒是最危险的情况,患者处于深昏迷状态,早期四肢肌张力增强、腱反射亢进、病理反射阳性,后期全身肌肉松弛、各种反射消失,呼吸不规则,甚至出现呼吸衰竭和休克。患者对外界事物和强烈刺激无反应,四肢冰冷,面色苍白,口唇紫绀,呼吸浅慢不规则,脉搏弱,血压下降,可有休克。
不同类型安眠药中毒的临床表现也有其特点。苯二氮卓类药物中毒的主要表现包括:中枢神经系统抑制,表现为嗜睡、昏睡甚至昏迷;呼吸抑制,呼吸频率减慢、呼吸浅弱,严重时可导致呼吸暂停;循环系统症状,如血压下降、心动过缓;胃肠道症状,如恶心、呕吐等;肝肾功能损害,可能出现转氨酶升高、肌酐升高等。
巴比妥类药物中毒的症状更为严重。轻度中毒时,患者有嗜睡、眩晕、头痛、语言迟钝、动作不协调等表现。中度中毒出现昏迷伴瞳孔缩小,但光反射存在。重度中毒特征为深昏迷伴呼吸浅慢、血压下降及反射消失。巴比妥类药物中毒的病死率较高,需要高度重视。
1.3 急诊快速识别要点
在急诊科,如何快速识别安眠药中毒患者?记住以下几个关键特征:
首先是意识状态的改变。约50%的患者在伤后1小时内出现意识障碍。从嗜睡到昏迷,程度不等。患者可能表现为嗜睡、意识模糊、昏睡甚至昏迷。
其次是呼吸系统症状。30%的患者出现呼吸困难。呼吸频率减慢,严重时出现呼吸暂停。这是安眠药中毒致死的主要原因之一。
第三是循环系统症状。20%的患者出现休克表现,包括血压下降、心率增快、皮肤苍白、四肢湿冷等。
第四是神经系统症状。患者可能出现头晕、共济失调、言语不清、眼球震颤等。严重者可出现抽搐、去大脑强直等。
第五是特殊的中毒线索。患者身边可能有药物空瓶或残留药物;有明确的服药过量史;可能伴有其他药物(如酒精)的同时摄入;患者或家属提供的病史提示药物过量。
在急诊快速评估时,还需要特别注意以下几点:
气道评估:确保气道通畅,注意有无呕吐物或分泌物阻塞气道。
呼吸评估:观察呼吸频率、节律和深度,监测血氧饱和度。呼吸频率低于12次/分或高于29次/分都是危险信号。
循环评估:监测血压、心率、脉搏血氧饱和度。收缩压低于90mmHg提示休克可能。
神经功能评估:进行GCS(格拉斯哥昏迷评分)评估,这是判断意识障碍程度的重要指标。
瞳孔检查:观察瞳孔大小、形状和对光反射。双侧瞳孔缩小可能提示巴比妥类中毒,而瞳孔散大固定则提示病情危重。
二、急诊处理的标准化流程
2.1 初始评估与生命支持
当安眠药中毒患者被送到急诊科时,我们必须遵循标准化的评估流程,确保不遗漏任何危及生命的情况。这个流程就是我们常说的ABCDE评估法。
A(Airway气道):首先评估气道是否通畅。如果患者GCS评分≤8分,或者存在气道梗阻的风险,如呕吐、意识不清等,应立即行气管插管,确保气道通畅。在操作过程中,要注意防止误吸,将患者头偏向一侧,及时清除口腔分泌物。
B(Breathing呼吸):评估呼吸功能,包括呼吸频率、节律、深度。使用脉搏血氧饱和度监测仪持续监测SpO2,目标是维持在95%以上。如果患者出现呼吸抑制,应立即给予高流量吸氧(4-6L/min),必要时使用呼吸机辅助呼吸。对于呼吸停止或严重呼吸抑制的患者,应立即进行人工呼吸或气管插管机械通气。
C(Circulation循环):评估循环功能,包括血压、心率、脉搏、皮肤颜色等。建立至少两条静脉通路,首选上肢大静脉。如果患者出现休克,应快速补液,使用生理盐水或林格氏液,初始量为20-30ml/kg。如果补液后血压仍不回升,可使用血管活性药物如多巴胺,初始剂量为2-5μg/kg/min,根据血压调整剂量。
D(Disability神经功能):进行快速神经功能评估,包括GCS评分、瞳孔检查、肢体活动等。GCS评分是国际通用的评估意识障碍程度的方法,包括睁眼反应、语言反应和运动反应三个部分,总分3-15分。评分越低,意识障碍越严重。
E(Exposure暴露):全面暴露患者身体,检查有无外伤、皮疹等异常。同时注意保暖,因为安眠药中毒患者可能出现低体温。
在完成ABCDE评估后,还需要进行以下紧急处理:
立即抽血检查:包括血常规、电解质、肝肾功能、血糖、血气分析等。特别要注意血糖水平,因为低血糖可能与安眠药中毒症状相似。
毒物检测:留取血、尿标本进行毒物筛查,明确中毒药物的种类和浓度。虽然药物浓度与临床症状的严重程度不一定成正比,但对于指导治疗和判断预后有重要意义。
心电监护:持续心电监护,注意有无心律失常。安眠药中毒可能导致各种心律失常,包括心动过缓、室性早搏等。
体温监测:每小时监测体温,因为严重中毒患者可能出现低体温。
2.2 清除毒物的方法与操作要点
清除毒物是安眠药中毒治疗的关键环节之一,目的是减少毒物的进一步吸收。常用的方法包括催吐、洗胃、活性炭吸附和导泻。
催吐的适应症与操作
催吐适用于意识清醒、能配合的患者,且服药时间在4-6小时以内。具体操作方法是:让患者先喝下300-500ml清水或温水,然后用压舌板或手指刺激咽喉部催吐,可反复几次,直到呕吐物为清水为止。
但是,催吐有严格的禁忌症:
• 意识不清或昏迷患者绝对不能催吐,因为可能导致误吸窒息
• 抽搐未控制的患者
• 有食管静脉曲张、胃溃疡等疾病的患者
• 服用腐蚀性毒物(如强酸、强碱)的患者
• 孕妇应慎用
催吐时要注意:动作要轻柔,避免损伤咽喉部;让患者保持侧卧位,头偏向一侧,防止呕吐物误吸;记录呕吐物的量和性状;如果患者出现呼吸困难、紫绀等情况,应立即停止催吐。
洗胃的操作规范
洗胃是清除胃内毒物最有效的方法,适用于服药时间在6小时以内的患者。但如果是缓释制剂或药物吸收缓慢,超过6小时仍可考虑洗胃。
洗胃的操作步骤:
1. 患者准备:患者取左侧卧位,头偏向一侧,头低脚高约15-30度,以防止呕吐物误吸。如果患者意识不清,应先进行气管插管保护气道。
2. 胃管选择:成人一般使用28-32号胃管(儿童用较细的胃管)。测量胃管插入长度:从鼻尖经耳垂到剑突的距离,一般为45-55cm。
3. 插入胃管:润滑胃管前端,经口腔缓慢插入。当胃管插入15cm时,嘱患者做吞咽动作,同时将胃管送下。如果患者昏迷,可将头后仰,插入胃管至预定长度。
4. 确认胃管位置:这一步非常重要!将胃管末端放入水中,观察有无气泡,如有气泡则说明胃管误入气管,需立即拔出重插。也可经胃管快速注入适量空气,在胃区听到咕噜声,证明胃管在胃内。
5. 洗胃过程:先抽尽胃内容物并保留送检。然后注入洗胃液200-300ml(儿童50-100ml),每次灌洗后尽量抽尽灌注液。反复灌洗,直至抽出液清亮、无味为止,一般需要2-10L洗胃液。
6. 洗胃液选择:
◦ 清水:是最常用的洗胃液,适用于毒物性质不明时
◦ 生理盐水:适用于有电解质紊乱的患者
◦ 2%碳酸氢钠:适用于有机磷农药中毒
◦ 1:5000高锰酸钾:适用于巴比妥类、阿片类药物中毒,但禁用于有机磷中毒
洗胃的注意事项:
• 每次灌入量不宜过多,以免胃内压过高导致毒物进入肠道或引起呕吐
• 保持灌入量与抽出量基本平衡,避免胃扩张
• 洗出液如为血性,应立即停止洗胃,并进行相应处理
• 洗胃过程中密切观察患者生命体征,如有异常立即停止
• 洗胃后可经胃管注入活性炭,以吸附肠道内残留毒物
洗胃的禁忌症:
• 吞服强酸、强碱等腐蚀性毒物
• 食管静脉曲张、食管狭窄
• 近期有上消化道出血、胃穿孔
• 严重心肺功能不全
• 昏迷患者如果未先行气管插管,也不能洗胃
活性炭的使用方法
活性炭是一种有效的吸附剂,能吸附多种安眠药,减少其在肠道的吸收。使用要点如下:
• 剂量:成人50-100g,儿童1g/kg,用250ml水调成糊状口服或经胃管注入
• 时机:应在洗胃后立即给予,越早使用效果越好
• 重复给药:对于长效药物或缓释制剂,可每4-6小时重复给药一次,但要注意防止便秘
• 注意事项:活性炭会影响某些药物(如抗生素)的吸收,如需使用应间隔2小时以上;肠梗阻患者禁用
导泻的方法
导泻的目的是加速肠道内毒物的排出。常用方法:
• 硫酸钠:成人20-30g,溶于200ml水中口服或胃管注入。硫酸钠在肠道内形成高渗状态,阻止水分吸收,促进肠蠕动。禁用硫酸镁,因为镁离子吸收后可加重中枢抑制
• 甘露醇:20%甘露醇250ml口服或胃管注入
• 聚乙二醇电解质溶液:成人1-2L/h,可有效导泻并保持水电解质平衡
导泻应在洗胃和活性炭使用后进行,一般不用于严重脱水、电解质紊乱或有肠梗阻的患者。
2.3 特效解毒剂氟马西尼的应用
氟马西尼是苯二氮卓类药物中毒的特效解毒剂,它是一种苯二氮卓受体拮抗剂,能竞争性阻断苯二氮卓类药物与受体结合,从而逆转其中枢抑制作用。
适应症与禁忌症
适应症:
• 明确的苯二氮卓类药物中毒
• 苯二氮卓类药物过量引起的中枢神经系统抑制
• 苯二氮卓类药物中毒导致的呼吸抑制
禁忌症(绝对禁用):
• 对氟马西尼过敏者
• 使用苯二氮卓类药物控制癫痫持续状态或颅内压者
• 严重抗抑郁剂中毒者
• 已知或怀疑为其他药物中毒(如三环类抗抑郁药)
• 长期使用苯二氮卓类药物的患者(可能诱发戒断症状)
慎用情况:
• 混合性药物中毒
• 肝功能不全者
• 头部损伤患者
• 药物或酒精依赖者
• 妊娠和哺乳期妇女
用法用量
氟马西尼的使用方法有严格要求,必须缓慢静脉注射,避免不良反应。
成人用法:
• 初始剂量:0.2mg,缓慢静脉注射(注射时间超过30秒)
• 如果30秒内未达到理想的清醒程度,可追加0.1mg,必要时可重复,直至患者清醒或总量达到1mg
• 维持剂量:清醒后如再次出现嗜睡,可给予0.1-0.4mg/h持续静脉滴注
对于苯二氮卓类药物过量的患者:
• 首次静脉注射剂量为0.3mg
• 如果60秒内未达到所需的清醒程度,可重复使用,直至患者清醒或总量达到2mg
• 如再度出现昏睡,可每小时静脉滴注0.1-0.4mg,根据清醒程度调整剂量
儿童用法:
• 推荐起始剂量为0.01mg/kg(最大不超过0.2mg),在15秒内静脉注射
• 若给药后未获得所需的意识水平,等待45秒后,可进一步注射0.01mg/kg(最大不超过0.2mg)
• 必要时以60秒的间隔重复注射(最多4次)
• 最大总剂量为0.05mg/kg或1mg,以较低者为准
使用注意事项
1. 给药速度:必须缓慢注射,避免快速给药导致的不良反应,如心律失常、高血压等。
2. 监测要点:
◦ 给药期间持续监测生命体征,特别是血压和心率
◦ 观察意识状态变化,记录清醒时间
◦ 注意有无戒断症状,如焦虑、震颤、抽搐等
◦ 监测呼吸功能,防止呼吸抑制反弹
3. 不良反应:
◦ 常见:恶心、呕吐、面色潮红
◦ 较少见:焦虑、心悸、恐惧、头晕
◦ 严重:心律失常、高血压、癫痫发作(特别是长期使用苯二氮卓类药物的患者)
4.特殊情况处理:
◦ 如果出现戒断症状(如焦虑、震颤),应立即停药,并给予地西泮5mg静脉注射
◦ 如果出现癫痫发作,可给予地西泮或苯妥英钠
◦ 如果出现严重高血压,可使用降压药物
5.治疗终点:
◦ 患者意识清醒,能正确回答问题
◦ 呼吸功能恢复正常
◦ 生命体征稳定
◦ 但要注意,氟马西尼的作用时间较短(约1小时),停药后可能再次出现镇静作用,需要继续观察
6.不适用情况:
◦ 不能用于巴比妥类或其他非苯二氮卓类安眠药中毒
◦ 不能作为诊断性用药(即不能用氟马西尼来判断是否为苯二氮卓类中毒)
◦ 不推荐用于长期使用苯二氮卓类药物的癫痫患者
2.4 支持治疗与并发症防治
支持治疗是安眠药中毒治疗的基础,贯穿整个治疗过程。主要包括以下几个方面:
呼吸支持
呼吸抑制是安眠药中毒最危险的并发症,也是致死的主要原因。处理措施包括:
1.保持呼吸道通畅:
◦ 将患者头偏向一侧,防止舌后坠
◦ 及时清除口腔分泌物
◦ 必要时放置口咽通气道
◦ 对于昏迷患者,应考虑气管插管
2.氧疗:
◦ 给予高流量吸氧(4-6L/min)
◦ 维持血氧饱和度在95%以上
◦ 根据血气分析结果调整氧浓度
3.机械通气:
◦ 指征:呼吸停止或严重呼吸抑制(呼吸频率<8次/分)
◦ 动脉血氧分压<60mmHg(吸氧状态下)
◦ 二氧化碳潴留(PaCO2>50mmHg)
◦ 呼吸节律异常,如潮式呼吸
4.呼吸兴奋剂:
◦ 可试用尼可刹米、洛贝林等
◦ 但效果有限,主要还是依靠机械通气
循环支持
循环衰竭是安眠药中毒的另一个严重并发症。处理措施:
1.液体复苏:
◦ 快速静脉补液,首选生理盐水
◦ 初始量:20-30ml/kg
◦ 根据血压、心率、尿量调整补液速度
◦ 监测中心静脉压(CVP),维持在5-12cmH2O
2.血管活性药物:
◦ 如果补液后血压仍低,可使用多巴胺
◦ 初始剂量:2-5μg/kg/min,根据血压调整
◦ 严重低血压可使用去甲肾上腺素
3.纠正心律失常:
◦ 心动过缓:可使用阿托品0.5-1mg静脉注射
◦ 室性心律失常:可使用利多卡因
◦ 密切监测心电图变化
水电解质平衡
安眠药中毒患者常伴有水电解质紊乱,需要及时纠正:
1.监测指标:
◦ 每4-6小时检查一次电解质
◦ 监测酸碱平衡
◦ 记录24小时出入量
2.常见问题及处理:
◦ 低钾血症:口服或静脉补钾,注意补钾速度
◦ 低钠血症:根据情况补充生理盐水或高渗盐水
◦ 脱水:根据脱水程度补充液体
◦ 酸中毒:轻度可通过补液纠正,严重者给予碳酸氢钠
体温管理
严重安眠药中毒患者可能出现低体温,处理措施:
• 监测体温,每小时一次
• 注意保暖,使用毛毯或升温毯
• 避免快速复温,防止心律失常
• 如果体温低于30℃,应考虑使用升温措施
预防并发症
1.肺部感染:
◦ 定时翻身、拍背,促进痰液排出
◦ 保持口腔清洁
◦ 必要时使用抗生素预防感染
◦ 监测体温变化,每4小时一次
2.深静脉血栓:
◦ 早期进行被动活动
◦ 使用弹力袜
◦ 病情允许时尽早下床活动
3.压疮:
◦ 定时翻身,每2小时一次
◦ 使用气垫床
◦ 保持皮肤清洁干燥
4.应激性溃疡:
◦ 使用质子泵抑制剂(如奥美拉唑)
◦ 观察大便颜色,必要时查潜血
5.肝肾功能损害:
◦ 监测肝肾功能
◦ 避免使用肾毒性药物
◦ 必要时进行血液净化治疗
营养支持
患者可能长时间不能进食,需要营养支持:
• 早期可通过鼻饲给予肠内营养
• 如不能耐受肠内营养,可考虑肠外营养
• 保证热量供应,一般为25-30kcal/kg/day
三、不同类型安眠药中毒的特异性处理
3.1 苯二氮卓类药物中毒
苯二氮卓类药物是目前临床最常用的安眠药,包括地西泮(安定)、艾司唑仑(舒乐安定)、阿普唑仑(佳静安定)、氯硝西泮、三唑仑等。这类药物的中毒处理有其特殊性。
中毒特点
1. 作用机制:苯二氮卓类药物通过增强GABA的抑制作用,降低神经元兴奋性,产生镇静、催眠、抗焦虑等作用。
2. 中毒表现:
◦ 中枢神经系统:从轻度镇静到深昏迷不等
◦ 呼吸抑制:严重时可致呼吸停止
◦ 心血管系统:血压下降、心动过缓
◦ 其他:共济失调、言语不清、眼球震颤等
3. 特殊表现:
◦ 三唑仑中毒:起效快,可突然出现昏迷,症状严重
◦ 氯硝西泮中毒:可能出现肌肉松弛、反射消失
◦ 长期使用者停药后可能出现戒断症状
特异性处理
1. 氟马西尼的使用:这是最重要的特效解毒剂,用法见前文。
2. 洗胃注意事项:
◦ 即使服药时间超过6小时,仍可考虑洗胃(因为部分药物吸收缓慢)
◦ 注意防止误吸,特别是三唑仑中毒患者
3. 血液净化:
◦ 血液透析对苯二氮卓类药物清除效果有限
◦ 血液灌流可能有效,但不是首选
◦ 主要还是依靠支持治疗
4. 戒断综合征处理:
◦ 长期使用苯二氮卓类药物的患者,突然停药可能出现戒断症状
◦ 表现为焦虑、震颤、失眠、抽搐等
◦ 处理:重新给予原药,然后逐渐减量停药
◦ 可使用长效苯二氮卓类药物(如地西泮)替代短效药物
3.2 巴比妥类药物中毒
巴比妥类药物虽然目前临床使用较少,但中毒后果严重,死亡率高,需要高度重视。包括苯巴比妥(鲁米那)、戊巴比妥、司可巴比妥等。
中毒特点
1. 作用机制:巴比妥类药物非选择性作用于GABA受体,延长氯离子通道开放时间,产生广泛的中枢抑制作用。
2. 中毒表现:
◦ 轻度:嗜睡、眩晕、头痛、言语不清
◦ 中度:昏迷、瞳孔缩小(光反射存在)、呼吸减慢
◦ 重度:深昏迷、瞳孔散大、呼吸停止、血压下降、休克
3.特殊表现:
◦ 体温降低(下丘脑体温调节中枢受抑制)
◦ 皮肤湿冷
◦ 可出现尿毒症
◦ 严重者可发生呼吸麻痹
特异性处理
1.洗胃:
◦ 即使超过6小时,仍应洗胃(因为巴比妥类药物吸收缓慢)
◦ 使用1:5000高锰酸钾溶液或生理盐水
2.碱化尿液:这是巴比妥类中毒的重要治疗措施!
◦ 原理:碱性环境可减少巴比妥类药物在肾小管的重吸收,加速排泄
◦ 方法:静脉滴注5%碳酸氢钠,使尿液pH值维持在7.5-8.5
◦ 监测:每2小时测一次尿pH值
◦ 注意:防止碱中毒和低钾血症
3.利尿剂:
◦ 可使用呋塞米(速尿)20-40mg静脉注射
◦ 但要注意维持水电解质平衡
4.血液净化:
◦ 血液透析对苯巴比妥清除效果好,是首选方法
◦ 血液灌流对短效巴比妥类效果更好
◦ 指征:严重中毒(血药浓度>80mg/L)、伴有肾衰、保守治疗无效
5.呼吸支持:
◦ 巴比妥类中毒的呼吸抑制往往很严重
◦ 早期气管插管,机械通气
◦ 使用呼吸兴奋剂效果有限
6.其他:
◦ 保温,防止低体温
◦ 预防感染
◦ 纠正酸中毒
3.3 新型安眠药中毒
随着药物研发的进展,新型安眠药不断涌现,它们的中毒特点和处理方法与传统药物有所不同。
唑吡坦(思诺思)中毒
1.中毒特点:
◦ 起效快(15-30分钟),作用时间短(3-6小时)
◦ 主要表现为深睡,但易唤醒
◦ 大剂量可导致昏迷、呼吸抑制
◦ 可能出现复杂的睡眠相关行为(如梦游、梦驾)
2.处理要点:
◦ 氟马西尼对唑吡坦中毒有效
◦ 支持治疗为主
◦ 注意:唑吡坦与酒精合用可导致严重的中枢抑制
佐匹克隆/右佐匹克隆中毒
1.中毒特点:
◦ 半衰期5-7小时
◦ 对入睡和睡眠维持障碍均有效
◦ 中毒症状相对较轻
◦ 可能出现口苦、头晕等副作用
2.处理要点:
◦ 主要是支持治疗
◦ 氟马西尼可能有效
◦ 注意监测肝功能
地达西尼中毒
地达西尼是2023年10月新列入管制的精神药品,属于新型苯二氮卓受体激动剂。目前关于其中毒的临床资料有限,但作为苯二氮卓类药物,其中毒处理原则与其他苯二氮卓类相似:
1.使用氟马西尼拮抗
2.支持治疗
3.必要时洗胃和活性炭吸附
特殊注意事项
1.药物相互作用:
◦ 新型安眠药与酒精合用可显著增加中枢抑制作用
◦ 与其他镇静药物(如抗组胺药)合用也需谨慎
2.个体差异:
◦ 老年人对新型安眠药更敏感
◦ 肝肾功能不全者药物半衰期延长
3.依赖性:
◦ 虽然新型安眠药的依赖性较传统药物低,但仍可能产生
◦ 长期使用后突然停药可能出现戒断症状
3.4 混合药物中毒的处理策略
在急诊实践中,我们经常遇到同时服用多种药物的患者,这增加了诊断和治疗的难度。混合药物中毒的特点是症状复杂、相互作用多、预后更差。
常见的混合中毒类型
1.安眠药+酒精:这是最常见的组合,两者有协同作用,可使中枢抑制作用增强2-5倍。据研究,65岁以上老年人中,因安眠药与酒精联用导致急性药物中毒的比例达到12.3%,其中超过一半为呼吸抑制导致的昏迷或死亡。
2.安眠药+抗抑郁药:特别是与三环类抗抑郁药合用,可增加心律失常和癫痫发作的风险。
3.安眠药+抗精神病药:如喹硫平、奥氮平等,可加重镇静作用。
4.多种安眠药混合:患者可能同时服用苯二氮卓类和巴比妥类药物,中毒症状更严重。
处理原则
1.快速评估:
◦ 详细询问服药史,包括药物种类、剂量、时间
◦ 检查身边的药瓶、残留药物
◦ 快速毒物筛查(虽然可能不全面)
◦ 重点评估生命体征,特别是呼吸和循环
2.支持治疗为主:
◦ 混合中毒时,特效解毒剂可能无效或有风险
◦ 以维持生命体征稳定为首要任务
◦ 呼吸支持:必要时机械通气
◦ 循环支持:补液、血管活性药物
◦ 纠正水电解质紊乱
3.慎用拮抗剂:
◦ 氟马西尼在混合中毒时要慎用
◦ 如果同时服用了其他可致惊厥的药物(如三环类抗抑郁药),使用氟马西尼可能诱发癫痫
◦ 必须使用时,应缓慢给药,并准备好抗惊厥药物
4.血液净化:
◦ 对于严重混合中毒,血液净化可能是有效的方法
◦ 可清除多种药物,特别是水溶性药物
◦ 但脂溶性药物(如地西泮)效果有限
5.对症处理:
◦ 癫痫发作:使用苯二氮卓类药物(如地西泮)
◦ 心律失常:根据类型选择药物
◦ 低血压:补液、血管活性药物
◦ 高热:降温治疗
6.观察时间:
◦ 混合中毒患者的观察时间应延长
◦ 因为不同药物的半衰期不同,可能出现症状反复
◦ 一般需要观察24-48小时,甚至更长
典型案例分析
一位62岁男性患者,因"意识不清4小时"入院。家属诉患者当晚与朋友聚餐时饮酒约200ml,回家后服用了"安眠药"(具体药物和剂量不详)。查体:深昏迷,GCS评分3分,呼吸浅慢(8次/分),血压80/50mmHg,心率50次/分,双侧瞳孔针尖样大小。
分析:
• 患者有明确的酒精和安眠药服用史
• 针尖样瞳孔提示可能有阿片类药物(如吗啡)中毒
• 但也可能是严重的中枢抑制
• 处理:立即气管插管,机械通气;快速补液,使用血管活性药物;洗胃(但要注意防止误吸);活性炭吸附;监测生命体征和血氧饱和度;毒物筛查
经验总结
1.混合中毒的诊断往往困难,要综合判断
2.治疗以支持为主,不要过分依赖解毒剂
3.要想到可能有未发现的药物(如非法药物)
4.观察要仔细,可能有延迟出现的症状
5.多学科协作很重要,必要时请精神科、毒理科会诊
四、特殊人群的处理要点
4.1 老年患者的特殊考虑
老年患者安眠药中毒有其独特的临床特点,需要特别关注。
老年患者的生理特点
1. 药代动力学改变:
◦ 肝肾功能减退,药物代谢和排泄减慢
◦ 药物半衰期延长,如地西泮的半衰期可从年轻人的20-30小时延长至70-100小时
◦ 血浆蛋白结合率改变,游离药物浓度增加
2. 药效学改变:
◦ 中枢神经系统对安眠药更敏感
◦ 同样剂量下,老年人的镇静作用更强
◦ 容易出现意识障碍和跌倒
3. 合并症多:
◦ 常合并高血压、冠心病、糖尿病等
◦ 同时服用多种药物,药物相互作用风险高
◦ 对药物副作用的耐受性差
临床表现特点
1. 症状不典型:
◦ 可能仅表现为嗜睡、精神萎靡
◦ 意识障碍程度可能与药物剂量不成比例
◦ 容易被误诊为其他疾病(如脑卒中、低血糖等)
2. 并发症多:
◦ 容易发生肺部感染
◦ 易出现水电解质紊乱
◦ 心血管并发症(心律失常、心力衰竭)
◦ 深静脉血栓形成风险高
3. 预后差:
◦ 老年人对药物的耐受性差
◦ 并发症发生率高
◦ 病死率较高
治疗要点
1. 评估要全面:
◦ 详细询问用药史,包括处方药、非处方药、保健品
◦ 了解基础疾病和用药情况
◦ 评估肝肾功能
2. 洗胃要谨慎:
◦ 老年人胃排空延迟,即使超过6小时仍可考虑洗胃
◦ 但要评估患者的耐受性
◦ 注意防止误吸和吸入性肺炎
3. 支持治疗要积极:
◦ 早期呼吸支持,不要等到出现严重呼吸抑制
◦ 维持循环稳定,避免使用强烈的血管活性药物
◦ 密切监测电解质,及时纠正
4.药物使用要谨慎:
◦ 氟马西尼使用要慎重,防止诱发戒断症状
◦ 避免使用可能加重意识障碍的药物
◦ 注意药物相互作用
5.预防并发症:
◦ 加强护理,定时翻身
◦ 预防肺部感染
◦ 预防深静脉血栓
◦ 预防压疮
6.观察时间要延长:
◦ 老年患者的恢复可能较慢
◦ 需要更长时间的观察
◦ 注意迟发并发症
4.2 儿童患者的处理要点
儿童安眠药中毒虽然相对少见,但一旦发生,后果严重,需要特别重视。
儿童的特点
1.药物敏感性高:
◦ 儿童的血脑屏障发育不完善,药物容易进入脑组织
◦ 对安眠药的敏感性高于成人
◦ 相同剂量下,中毒症状可能更严重
2.表达能力有限:
◦ 婴幼儿不能诉说不适
◦ 学龄前儿童可能说不清服药情况
◦ 需要家长提供准确病史
3.病情变化快:
◦ 儿童的病情变化迅速
◦ 可能在短时间内从轻度中毒发展到重度
4.误服多见:
◦ 多为误服家长的药物
◦ 药物存放不当是主要原因
临床表现特点
1.症状不典型:
◦ 可能表现为嗜睡、不愿进食
◦ 烦躁不安、哭闹
◦ 呼吸不规则
◦ 体温降低
2.易发生并发症:
◦ 呼吸暂停
◦ 吸入性肺炎
◦ 低血糖
◦ 脑水肿
治疗要点
1.快速评估:
◦ 评估意识状态(使用儿童GCS评分)
◦ 评估气道、呼吸、循环
◦ 询问病史(重点是药物种类和剂量)
2.洗胃注意事项:
◦ 儿童洗胃的风险更高
◦ 胃管选择要合适(较细)
◦ 洗胃液量要控制(每次50-100ml)
◦ 防止胃扩张和误吸
◦ 必要时先气管插管保护气道
3.活性炭使用:
◦ 剂量:1g/kg
◦ 可重复使用,但要防止便秘
◦ 注意:6个月以下婴儿慎用
4.氟马西尼使用:
◦ 儿童可以使用,但剂量要精确计算
◦ 起始剂量:0.01mg/kg
◦ 最大剂量:1mg
◦ 缓慢注射,密切观察
5.支持治疗:
◦ 维持正常体温,防止低体温
◦ 监测血糖,防止低血糖(儿童糖原储备少)
◦ 维持水电解质平衡
◦ 预防感染
6.特殊注意事项:
◦ 防止医源性损伤(如静脉穿刺)
◦ 注意保暖
◦ 安抚家长情绪
◦ 考虑是否为虐待或忽视
7.预防宣教:
◦ 药物要妥善保管,放在儿童不能触及的地方
◦ 不要将药物放在饮料瓶中
◦ 教育儿童不要随便吃药
4.3 妊娠患者的处理要点
妊娠患者安眠药中毒是一个特殊情况,处理时需要兼顾母胎安全。
妊娠患者的特点
1.生理变化:
◦ 血容量增加,药物分布容积改变
◦ 肝肾功能改变,药物代谢和排泄变化
◦ 血浆蛋白水平降低,游离药物浓度增加
2.对胎儿的影响:
◦ 药物可通过胎盘屏障
◦ 可能导致胎儿畸形
◦ 可能引起早产或流产
◦ 新生儿可能出现戒断症状
3.临床表现:
◦ 与非妊娠患者相似
◦ 但要特别关注胎儿情况
◦ 可能出现宫缩
治疗要点
1.快速评估:
◦ 评估孕妇的生命体征
◦ 评估胎儿情况(胎心监护)
◦ 确定妊娠周数
2.支持治疗:
◦ 维持孕妇生命体征稳定
◦ 给氧,维持血氧饱和度>95%
◦ 补液,维持循环稳定
◦ 监测胎儿心率(每30分钟一次)
3.清除毒物:
◦ 洗胃要慎重,特别是孕早期
◦ 如果必须洗胃,应在气管插管保护下进行
◦ 活性炭可以使用,但要注意剂量
◦ 避免使用导泻剂,防止诱发宫缩
4.氟马西尼使用:
◦ 妊娠早期(前3个月)禁用
◦ 妊娠中晚期慎用
◦ 如果使用,要密切监测胎儿情况
5.避免使用的药物:
◦ 巴比妥类:可通过胎盘,对胎儿有害
◦ 苯二氮卓类:孕早期使用可能导致胎儿唇裂、腭裂
◦ 其他中枢抑制剂
6.产科处理:
◦ 请产科医生会诊
◦ 监测宫缩,必要时使用宫缩抑制剂
◦ 做好早产准备
◦ 准备新生儿复苏
7.产后注意事项:
◦ 新生儿可能出现戒断症状(烦躁、震颤、喂养困难)
◦ 需要儿科医生密切观察
◦ 可能需要药物治疗
◦ 母亲如果长期使用安眠药,应避免母乳喂养
4.4 慢性中毒与戒断综合征
除了急性中毒,我们还需要了解慢性中毒和戒断综合征的处理。
慢性中毒
长期使用安眠药的患者可能出现慢性中毒,表现为:
1.精神症状:
◦ 认知功能障碍
◦ 记忆力减退
◦ 情绪不稳定
◦ 人格改变
2.躯体症状:
◦ 嗜睡、乏力
◦ 步态不稳
◦ 震颤
◦ 性功能障碍
3.处理原则:
◦ 逐渐减量停药,避免突然停药
◦ 可使用长效药物替代短效药物
◦ 如地西泮替代三唑仑
◦ 给予支持治疗,包括营养支持
◦ 心理治疗,帮助患者克服依赖
戒断综合征
长期使用安眠药的患者突然停药或减量过快,可能出现戒断综合征:
1.症状表现:
◦ 反跳性失眠(比原来更严重)
◦ 焦虑、烦躁
◦ 震颤、出汗
◦ 恶心、呕吐
◦ 癫痫发作(严重时)
◦ 谵妄(罕见但严重)
2.处理方法:
◦ 重新给予原药,症状缓解后再缓慢减量
◦ 减量速度要慢,一般每2-4周减少原剂量的10-20%
◦ 可使用辅助药物:
▪ β受体阻滞剂(如普萘洛尔)缓解震颤、心悸
▪ 抗抑郁药(如SSRI类)治疗焦虑、抑郁
▪ 抗惊厥药物预防癫痫
3.预防措施:
◦ 严格掌握安眠药使用指征,避免长期使用
◦ 需要长期使用时,应定期评估
◦ 停药时要逐渐减量
◦ 告知患者停药可能出现的症状
五、最新临床指南与研究进展
5.1 2023-2025年国际指南更新要点
近年来,安眠药中毒的治疗理念发生了重要变化,国际权威机构发布的指南也进行了相应更新。
美国临床毒理学会(AACT)指南更新(2023年)
1. 洗胃时机的新认识:
◦ 传统认为中毒后6小时内洗胃有效,但新指南指出,对于缓释制剂或脂溶性药物,即使超过6小时仍可考虑洗胃
◦ 重点不是时间,而是药物是否仍在胃内
◦ 建议在摄入药物后1-2小时内洗胃效果最佳
2. 活性炭使用的优化:
◦ 推荐剂量:成人50-100g,儿童1g/kg
◦ 对于缓释制剂或有肠肝循环的药物,可重复给药(每4-6小时一次)
◦ 不推荐常规使用泻药,因为可能加重电解质紊乱
3. 氟马西尼使用原则:
◦ 明确指出不能作为诊断性用药
◦ 不推荐用于混合药物中毒,特别是可能含有致惊厥药物时
◦ 对于长期使用苯二氮卓类药物的患者,使用时要极其谨慎
4. 支持治疗的强化:
◦ 强调早期呼吸支持的重要性
◦ 建议对所有中重度中毒患者进行心电监护
◦ 维持正常体温,低体温会影响药物代谢
欧洲中毒中心和临床毒理学家协会(EAPCCT)指南(2024年)
1. 风险分层评估:
◦ 轻度中毒:GCS 13-15分,生命体征稳定
◦ 中度中毒:GCS 9-12分,需要密切监测
◦ 重度中毒:GCS ≤8分,需要立即生命支持
2. 血液净化的适应症:
◦ 血液透析:适用于苯巴比妥中毒,特别是血药浓度>80mg/L
◦ 血液灌流:适用于脂溶性药物(如地西泮)
◦ 不推荐用于苯二氮卓类药物中毒常规治疗
3. 新型解毒剂的探索:
◦ 目前尚无针对新型安眠药(如唑吡坦)的特效解毒剂
◦ 氟马西尼对部分新型安眠药可能有效,但证据有限
中国急诊医学学会专家共识(2025年)
1. 分级诊疗体系:
◦ 基层医院:主要进行初步评估、稳定生命体征、联系转诊
◦ 二级医院:可进行洗胃、活性炭吸附、支持治疗
◦ 三级医院:具备血液净化、重症监护等条件
2. 院前急救要点:
◦ 保持气道通畅,必要时现场气管插管
◦ 持续监测生命体征
◦ 尽快转运至有条件的医院
3.院内救治流程:
◦ 建立标准化救治流程
◦ 多学科协作(急诊、ICU、毒理、精神科)
◦ 强调个体化治疗
5.2 新的治疗方法与技术
随着医学技术的发展,一些新的治疗方法正在临床应用或研究中。
血液净化技术的进展
1.连续肾脏替代治疗(CRRT):
◦ 对于血流动力学不稳定的患者,CRRT是更好的选择
◦ 可以缓慢、持续地清除毒物
◦ 同时维持水电解质平衡
2.血浆置换:
◦ 适用于与蛋白结合率高的药物中毒
◦ 可以清除血浆中的药物和毒素
◦ 但需要大量血浆,成本较高
3.分子吸附再循环系统(MARS):
◦ 可以清除蛋白结合的药物
◦ 对脂溶性药物可能有效
◦ 但技术要求高,费用昂贵
新型解毒剂的研发
1.GABA受体调节剂:
◦ 正在研究开发新的GABA受体拮抗剂
◦ 可能对多种安眠药中毒有效
◦ 但目前还处于实验阶段
2.特异性抗体:
◦ 针对特定药物的单克隆抗体
◦ 可以快速中和药物毒性
◦ 但制备困难,成本极高
人工智能在中毒诊断中的应用
1.辅助诊断系统:
◦ 通过机器学习分析中毒症状
◦ 快速识别可能的毒物
◦ 提高诊断准确率
2.治疗决策支持:
◦ 根据患者情况推荐治疗方案
◦ 预测治疗效果
◦ 优化治疗流程
超声技术的应用
1.胃内药物检测:
◦ 超声可以检测胃内是否有药物残留
◦ 判断洗胃效果
◦ 指导是否需要继续洗胃
2.容量评估:
◦ 超声评估下腔静脉直径
◦ 判断容量状态
◦ 指导补液治疗
5.3 临床研究的重要发现
近年来的临床研究为安眠药中毒的治疗提供了新的证据。
氟马西尼持续输注的研究
2025年发表在《MDPI》杂志的一项回顾性研究显示,对于严重苯二氮卓类中毒患者,持续静脉输注氟马西尼可以加快意识恢复。研究纳入了120例患者,分为持续输注组和间断给药组,结果显示:
• 持续输注组意识恢复时间平均为2.5小时,间断给药组为4.8小时
• 两组不良反应发生率相似(约15%)
• 建议持续输注剂量为0.1-0.4mg/h
安眠药与阿尔茨海默病关系的研究
2025年《Cell》期刊发表的重磅研究揭示了安眠药与阿尔茨海默病之间的潜在联系:
• 唑吡坦等安眠药会抑制睡眠期间大脑的废物清除功能
• 导致β淀粉样蛋白等有毒物质堆积
• 长期使用安眠药者认知功能下降速度比常人快30%
• 小胶质细胞异常激活,过度吞噬神经元突触
褪黑素与心力衰竭风险的研究
2024年美国心脏协会科学会议上发布的研究显示:
• 使用褪黑素的患者心力衰竭风险增加89%
• 长期使用(超过1年)风险增加90%
• 死亡风险增加近2倍
新型安眠药的中毒案例
1.苏沃雷生(suvorexant)中毒:
◦ 这是一种食欲素受体拮抗剂
◦ 中毒可导致严重的低温
◦ 一例与佐匹克隆混合中毒导致死亡的案例显示,药物相互作用可导致致命性低温
2.美托咪定(medetomidine)中毒:
◦ 这是一种α2受体激动剂,用于动物麻醉
◦ 比甲苯噻嗪(xylazine)强200-300倍
◦ 已在多个国家报告中毒案例,表现为深度镇静、心动过缓、低血压
5.4 急诊科医生的经验总结
基于大量临床实践,急诊科医生总结了以下经验:
快速识别要点
1.病史采集技巧:
◦ 询问患者时要简单直接,如"你吃了什么药?吃了多少?"
◦ 检查患者随身物品,可能发现药瓶
◦ 询问家属时要注意,他们可能隐瞒药物种类
◦ 注意患者的用药习惯,是否有药物依赖
2.快速判断中毒程度:
◦ GCS评分是最可靠的指标
◦ 呼吸频率<12次/分或>29次/分提示严重中毒
◦ 血压<90/60mmHg提示休克
◦ 瞳孔变化有助于判断药物类型
3.常见误区:
◦ 不要被"患者看起来清醒"误导,可能是假象
◦ 老年人的症状可能不典型
◦ 混合中毒时症状可能相互掩盖
救治经验
15.洗胃时机的把握:
◦ 宁早勿晚,特别是清醒患者
◦ 如果患者不配合,可先给予镇静剂(如小剂量地西泮),然后洗胃
◦ 但要注意,镇静剂可能加重病情
2.氟马西尼使用经验:
◦ 先给小剂量试验(0.1mg),观察反应
◦ 如果患者清醒,说明是苯二氮卓类中毒
◦ 但要准备好抢救设备,防止戒断反应
◦ 对于长期使用者,宁可不用
3.特殊情况处理:
◦ 患者不配合:必要时约束,但要注意安全
◦ 家属不理解:耐心解释,必要时请上级医生沟通
◦ 怀疑自杀:同时请精神科会诊,防止再次自杀
4.并发症预防经验:
◦ 早期使用质子泵抑制剂预防应激性溃疡
◦ 定时翻身,预防压疮
◦ 尽早活动,预防深静脉血栓
◦ 加强口腔护理,预防感染
5.成功救治案例分析:
案例一:一位35岁女性,误服地西泮50片(5mg/片),约2小时后被发现。入院时嗜睡,GCS 13分。立即洗胃,洗出大量未溶解药片。给予活性炭,静脉补液。观察6小时后患者清醒,无后遗症。
经验:早期洗胃非常重要,及时清除了胃内药物。
案例二:一位65岁男性,长期服用阿普唑仑,因失眠自行加量至20片/天,3天后出现意识模糊。考虑慢性中毒合并急性加重。给予支持治疗,逐渐减量药物,2周后症状缓解。
经验:对于长期用药患者,不能突然停药,要逐渐减量。
6.失败教训总结:
教训一:一位22岁女性,服用地西泮100片后昏迷,家属未及时发现,6小时后才送医。虽经积极抢救,包括血液灌流,但患者仍因多器官功能衰竭死亡。
教训:时间就是生命,早发现、早治疗至关重要。
教训二:一位患者同时服用地西泮和阿米替林(抗抑郁药),医生误用氟马西尼,导致癫痫发作,最终死亡。
教训:混合中毒时慎用氟马西尼,特别是可能有其他致惊厥药物时。
7.实用技巧:
◦ 准备一个"中毒急救包",包含常用解毒剂、洗胃用品等
◦ 建立毒物信息库,方便查询
◦ 与毒物控制中心建立联系,随时咨询
◦ 定期培训,提高团队救治能力
六、总结与展望
安眠药中毒是急诊科常见的急症,掌握正确的诊断和治疗方法对挽救患者生命至关重要。通过本指南的学习,我们可以总结出以下要点:
关键知识点回顾
1. 分类与特点:
◦ 安眠药主要分为苯二氮卓类、巴比妥类和新型安眠药
◦ 不同类型安眠药中毒的临床表现和处理方法不同
◦ 混合药物中毒和老年患者预后较差
2. 诊断要点:
◦ 详细询问病史,包括药物种类和剂量
◦ 快速评估意识状态(GCS评分)
◦ 重点关注呼吸和循环功能
◦ 注意与其他疾病鉴别
3. 治疗原则:
◦ ABCDE评估是基础,确保生命体征稳定
◦ 清除毒物:催吐、洗胃、活性炭、导泻
◦ 特效解毒剂:氟马西尼(仅用于苯二氮卓类)
◦ 支持治疗:呼吸支持、循环支持、水电解质平衡
◦ 预防并发症
4. 特殊人群:
◦ 老年人:药代动力学改变,耐受性差
◦ 儿童:病情变化快,需要特殊护理
◦ 妊娠患者:兼顾母胎安全,慎用药物
◦ 慢性中毒:逐渐减量,预防戒断
5. 最新进展:
◦ 洗胃时机:不严格限制在6小时内
◦ 血液净化:CRRT更适合血流动力学不稳定患者
◦ 新型药物:关注新型安眠药的中毒特点
◦ 人工智能:辅助诊断和治疗决策
未来展望
随着医学技术的不断进步,安眠药中毒的救治将朝着以下方向发展:
1. 精准医疗:
◦ 根据患者基因型制定个体化治疗方案
◦ 快速毒物检测技术,实现精准诊断
◦ 开发特异性解毒剂
2. 人工智能应用:
◦ AI辅助诊断系统,提高诊断准确率
◦ 智能监测设备,实时评估病情变化
◦ 远程医疗,获得专家指导
3. 新治疗方法:
◦ 纳米技术:开发新型解毒剂载体
◦ 基因治疗:修复药物代谢酶缺陷
◦ 细胞治疗:干细胞修复受损组织
4. 预防体系建设:
◦ 加强安眠药处方管理,防止滥用
◦ 开展公众健康教育,提高安全用药意识
◦ 建立药物不良反应监测系统
给急诊科医生的建议
1.提高警惕性:
◦ 对所有意识障碍患者,都要考虑中毒可能
◦ 不要被表面现象迷惑,深入了解病史
◦ 想到最严重的情况,做好充分准备
2.规范操作:
◦ 严格按照流程操作,不遗漏任何步骤
◦ 注意细节,如胃管位置确认、洗胃液温度等
◦ 做好记录,包括时间、剂量、患者反应
3.团队协作:
◦ 与护士、药师密切配合
◦ 及时请相关科室会诊
◦ 建立良好的沟通机制
4.持续学习:
◦ 关注最新指南和研究进展
◦ 参加培训和学术会议
◦ 总结经验教训,不断提高
5.人文关怀:
◦ 理解患者和家属的心理
◦ 耐心解释病情和治疗方案
◦ 关注患者的心理健康,预防再次自杀
最后的话
安眠药中毒虽然常见,但每一个患者都是独特的,需要我们用心去救治。记住,在急诊工作中:
• 时间就是生命,早一秒救治,就多一分希望
• 冷静分析,不要被慌乱情绪影响判断
• 团队协作,发挥每个人的专业优势
• 不断学习,用最新的知识武装自己
作者提示: 个人观点,仅供参考
转自:白大褂的日记
【版权声明】 图文及视听资料来源于网络,在此致谢!版权归原作者所有,所有内容仅供专业人士交流分享,如有侵权,请联系删除。谢谢! 投稿、合作与联络微信:dengyl2401。
2024-12-11
WEDNESDAY, Dec. 11, 2024 -- Adults receiving chiropractic spinal manipulative therapy (SMT) for tension-type headache have a significantly lower likelihood of butalbital prescription, according to a study published online Nov. 29 in
Health Science Reports
.
Robert J. Trager, D.C., from the Case Western Reserve University School of Medicine in Cleveland, and colleagues assessed whether receiving chiropractic SMT for tension-type headache impacts receipt of butalbital prescription. The analysis included 3,116 matched patients with tension-type headache receiving and not receiving SMT.
The researchers found that the incidence of butalbital prescription was lower in the SMT cohort versus the non-SMT cohort (SMT: 1.7 percent; non-SMT: 3.8 percent; risk ratio, 0.46). The SMT cohort also had a lower incidence of medication overuse headache versus the non-SMT cohort (SMT: 0.5 percent; non-SMT: 1.2 percent; risk ratio, 0.44).
"Adults receiving chiropractic SMT had a significantly lower likelihood of butalbital prescription and, tentatively, medication overuse headache compared to matched controls not receiving SMTs," the authors write. "These findings reinforce clinical practice guidelines already recommending SMT for tension-type headache. However, additional research is needed to corroborate our results and examine the association between a broader variety of nonpharmacologic interventions and butalbital prescription and medication overuse headache."
Abstract/Full Text
Whatever your topic of interest,
subscribe to our newsletters
to get the best of Drugs.com in your inbox.
临床结果
2022-08-31
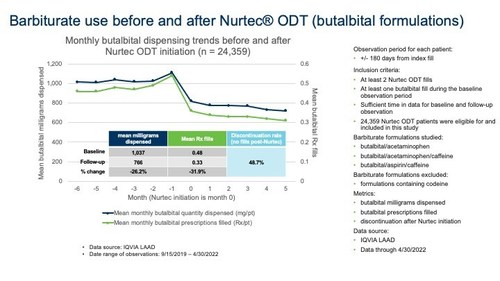
Results from this study demonstrate a robust and clinically meaningful reduction in barbiturate use after the initiation of Nurtec ODT, providing an alternative migraine therapy for those who may be reliant on butalbital or desire an alternative therapy that is not associated with addiction potential.
Despite being associated with addiction potential, medication overuse (rebound) headaches and central nervous system side effects, barbiturates are prescribed as first-line migraine treatments.
Longitudinal medical and prescription claims were used to assess barbiturate prescriptions and mean milligrams dispensed amongst migraine patients observed 6 months prior to and following Nurtec ODT initiation.
Among the 24,359 with migraine who used butalbital prior to initiating treatment with Nurtec ODT, approximately 49% had no butalbital prescription fills in the 6 months following initiation.
NEW HAVEN, Conn., Aug. 31, 2022 /PRNewswire/ -- Biohaven Pharmaceutical Holding Company Ltd. (NYSE: BHVN) announced the important findings pertaining to the benefit of Nurtec® ODT (rimegepant) in decreasing the burden of butalbital use among migraine patients in real world clinical practice.
Continue Reading
Figure 1
Noah Rosen, M.D., Northwell Physician Partners, Neuroscience Institute of Great Neck, NY commented, "Butalbital containing compound prescriptions remain high despite longstanding questions of safety in other conditions, significant potential side effects, multiple drug interactions and risk of dependency and misuse." Neurologist, Katherine Standley D.O., Medical Director, Biohaven, commented "Butalbital has a high risk of leading to medication overuse headaches, and has led to the American Academy of Neurology (AAN) and the American Headache Society (AHS) to recommend avoiding its use as a first line agent in the treatment of headaches."
The study population was derived from a longitudinal and anonymized integrated commercial medical and prescription claims database from September 15, 2019 through April 30, 2022. The data contains patient-level claims with plan, payer, facility, procedure, medication, and diagnosis information. The observation period for each patient was +/- 180 days from index fill. To be included in the study, the patient had to have filled at least 2 Nurtec ODT prescriptions, have at least one butalbital fill during the baseline observation period, and have sufficient time in data for baseline and follow-up observation. Barbiturate formulations studied included butalbital/acetaminophen, butalbital/acetaminophen/caffeine, and butalbital/aspirin/caffeine. Barbiturate formulations containing codeine were excluded.
Among 491,149 Nurtec patients, 24,359 met the inclusion criteria and are described in Figure 1 below. The overall butalbital discontinuation rate after Nurtec ODT initiation was 48.7%. Overall, mean monthly butalbital prescription fills decreased by 31.9% and mean milligrams dispensed reduced by 26.2%.
Gil L'Italien Ph.D., Senior Vice President, GHEOR & Epidemiology, Biohaven, commented, "Despite treatment guidelines suggesting that barbiturates should be avoided for treatment of migraine as the first line due to addiction potential, central nervous system effects and medication overuse headaches, barbiturate is prescribed in clinical practice, sometimes as first line in patients who are not candidates for triptans. Administrative claims provide a rich source of data on treatment patterns associated with the introduction of novel medications, and the duration of follow-up affords the opportunity to assess trends associated with these transitions. Our findings support the benefit of Nurtec ODT as an effective and safe migraine treatment that can reduce the need for barbiturates."
Dr. Rosen further commented, "This study has given a real-world insight into the impact that offering a more specific treatment can have on driving medication use away from older nonspecific remedies. Furthermore, by initiating Nurtec ODT, not only did patients lower butalbital use, but also reduced concurrent caffeine, acetaminophen or aspirin use due to their presence in these combination analgesics."
Dr. Standley added, "Prior to the institution of CGRP antagonists, like Nurtec ODT, we had limited options for acute management of migraine in patients who were intolerant or not candidates for triptan therapies. Medications such as opioids and barbiturate containing analgesics are associated with addiction potential and increased risk of chronic migraine. This data supports that Nurtec ODT initiation is associated with a meaningful reduction in butalbital use in real-world data. When coupled with the previously presented opioid data, this suggests an overall reduction in the use of controlled substances with the initiation of Nurtec ODT."
NURTEC ODT (rimegepant) was approved by the U.S. Food and Drug Administration (FDA) for the acute treatment of migraine in February 2020 and for the preventive treatment of episodic migraine in May 2021.
About NURTEC ODT
NURTEC ODT (rimegepant) is the first and only calcitonin gene-related peptide (CGRP) receptor antagonist available in a quick-dissolve ODT formulation that is approved by the U.S. Food and Drug Administration (FDA) for the acute treatment of migraine with or without aura and the preventive treatment of episodic migraine in adults. The activity of the neuropeptide CGRP is thought to play a causal role in migraine pathophysiology. NURTEC ODT is a CGRP receptor antagonist that works by reversibly blocking CGRP receptors, thereby inhibiting the biologic activity of the CGRP neuropeptide. The recommended dose of NURTEC ODT is 75 mg, taken as needed, up to once daily to treat or every other day to help prevent migraine attacks. For more information about NURTEC ODT, visit .
Indication
NURTEC ODT orally disintegrating tablets is a prescription medicine that is used to treat migraine in adults. It is for the acute treatment of migraine attacks with or without aura and the preventive treatment of episodic migraine. It is not known if NURTEC ODT is safe and effective in children.
Important Safety Information
Do not take NURTEC ODT if you are allergic to NURTEC ODT (rimegepant) or any of its ingredients.
Before you take NURTEC ODT, tell your healthcare provider (HCP) about all your medical conditions, including if you:
have liver problems,
have kidney problems,
are pregnant or plan to become pregnant,
are breastfeeding or plan to breastfeed.
Tell your HCP about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
NURTEC ODT may cause serious side effects including allergic reactions, trouble breathing and rash. This can happen days after you take NURTEC ODT. Call your HCP or get emergency help right away if you have swelling of the face, mouth, tongue, or throat or trouble breathing. This occurred in less than 1% of patients treated with NURTEC ODT.
The most common side effects of NURTEC ODT were nausea (2.7%) and stomach pain/indigestion (2.4%). These are not the only possible side effects of NURTEC ODT. Tell your HCP if you have any side effects.
You are encouraged to report side effects of prescription drugs to the FDA.
Visit or call 1–800–FDA–1088 or report side effects to Biohaven at 1–833–4NURTEC.
See full Prescribing Information and Patient Information.
About Migraine
Nearly 40 million people in the U.S. suffer from migraine and the World Health Organization classifies migraine as one of the 10 most disabling medical illnesses. Migraine is characterized by debilitating attacks lasting four to 72 hours with multiple symptoms, including pulsating headaches of moderate to severe pain intensity that can be associated with nausea or vomiting, and/or sensitivity to sound (phonophobia) and sensitivity to light (photophobia). There is a significant unmet need for new treatments as more than 90 percent of people with migraine are unable to work or function normally during an attack.
CGRP Receptor Antagonism
Small molecule CGRP receptor antagonists represent a novel class of drugs for the treatment of migraine. CGRP receptor antagonists work by reversibly blocking CGRP receptors, thereby inhibiting the biologic activity of the CGRP neuropeptide. For acute treatment, this unique mode of action potentially offers an alternative to other agents, particularly for patients who have contraindications to the use of triptans or who have a poor response to triptans or are intolerant to them. CGRP signal-blocking therapies have not been associated with medication overuse headache (MOH) or rebound headaches which limits the clinical utility of other acute treatments due to increases in migraine attacks that result from frequent use.
About Biohaven
Biohaven is a global commercial-stage biopharmaceutical company with a portfolio of innovative, best-in-class therapies to improve the lives of patients with debilitating neurological and neuropsychiatric diseases, including rare disorders. Biohaven's Neuroinnovation™ portfolio includes FDA-approved Nurtec ODT (rimegepant) for the acute and preventive treatment of migraine EMA-approved as Vydura® for the acute treatment of migraine with or without aura, and prophylaxis of episodic migraine in adults who have at least four migraine attacks per month) and a broad pipeline of late-stage product candidates across five distinct mechanistic platforms: CGRP receptor antagonism for the acute and preventive treatment of migraine; glutamate modulation for obsessive-compulsive disorder and spinocerebellar ataxia; and MPO inhibition for amyotrophic lateral sclerosis; Kv7 Ion Channel Activators (Kv7) activators for focal epilepsy and neuronal hyperexcitability, and myostatin inhibition for neuromuscular diseases. More information about Biohaven is available at .
Forward-Looking Statements
This news release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements involve substantial risks and uncertainties, including statements that are based on the current expectations and assumptions of Biohaven's management about NURTEC ODT as an acute treatment for patients with migraine and preventive treatment for migraine. Factors that could affect these forward-looking statements include those related to: Biohaven's ability to effectively commercialize NURTEC ODT, delays or problems in the supply or manufacture of NURTEC ODT, complying with applicable U.S. regulatory requirements, the expected timing, commencement and outcomes of Biohaven's planned and ongoing clinical trials; the timing of planned interactions and filings with the FDA; the timing and outcome of expected regulatory filings; the potential commercialization of Biohaven's product candidates; the potential for Biohaven's product candidates to be first in class or best in class therapies; and the effectiveness and safety of Biohaven's product candidates. Various important factors could cause actual results or events to differ materially from those that may be expressed or implied by forward-looking statements. Additional important factors to be considered in connection with forward-looking statements are described in the "Risk Factors" section of Biohaven's Annual Report on Form 10-K filed with the Securities and Exchange Commission on February 25, 2022, and in Biohaven's subsequent filings with the Securities and Exchange Commission. The forward-looking statements are made as of the date of this release, and Biohaven does not undertake any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
NURTEC and NURTEC ODT are registered trademarks of Biohaven Pharmaceutical Ireland DAC. Neuroinnovation is a trademark of Biohaven Pharmaceutical Holding Company Ltd.
Biohaven Contact
Jennifer Porcelli
Vice President, Investor Relations
[email protected]
201-248-0741
Media Contact
Mike Beyer
Sam Brown Inc.
[email protected]
312-961-2502
SOURCE Biohaven Pharmaceutical Holding Company Ltd.
First in ClassBest in Class小分子药物
100 项与 布他比妥 相关的药物交易
登录后查看更多信息
研发状态
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
No Data | |||||||
登录后查看更多信息
转化医学
使用我们的转化医学数据加速您的研究。
登录
或

药物交易
使用我们的药物交易数据加速您的研究。
登录
或

核心专利
使用我们的核心专利数据促进您的研究。
登录
或

临床分析
紧跟全球注册中心的最新临床试验。
登录
或

批准
利用最新的监管批准信息加速您的研究。
登录
或

特殊审评
只需点击几下即可了解关键药物信息。
登录
或

生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用