预约演示
IlluminOss announces their 200th patient in their global device registry, studying the effectiveness and patient outcomes of their revolutionary device for addressing bone fractures
2024-02-06
EAST PROVIDENCE, R.I., Feb. 6, 2024 /PRNewswire/ -- IlluminOss Medical, offering a unique, minimally invasive technology for fracture repair and stabilization in geriatric and compromised bone, announced it has reached the important benchmark of enrolling over 200 patients in its global device registry across 16 sites. IlluminOss will highlight the results of the patients in the registry at the upcoming American Academy of Orthopedic Surgeons meeting.
Continue Reading
Preview
来源: PRNewswire
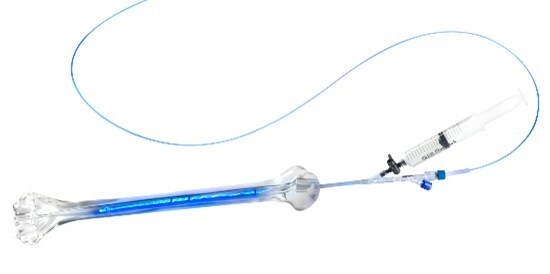
IlluminOss Photodynamic Bone Stabilization System
To further the evidence of the 1000+ patients' data in peer reviewed publications and conference proceedings, IlluminOss launched a clinical registry to demonstrate the real-world use of our device. The registry is designed to collect safety and performance data on the IlluminOss Device when used to provide stabilization and alignment for the treatment of fractures in compromised bone. The registry aims to show real world outcomes for the system. Standard of care follow up visit data is collected at 75 days, 6 months, and 1 year from the initial procedure. Over half of the patients enrolled have already reached their first follow up visit.
About the IlluminOss Photodynamic Bone Stabilization System
IlluminOss technology involves expanding a balloon-like implant inside a fractured bone by infusing the implant with a light-curing liquid. Blue light is then transmitted to the implant via an optical fiber to convert the liquid into a polymer, creating a strong core within the bone. This stabilizes the fracture and allows for a fast return to daily activities. Some patients are able to regain use of their injured limb the day of surgery. If additional fracture fixation is necessary, the strength supplied by the IlluminOss implant allows surgical plates and screws to be securely fastened into the bone.
Dr. Jason Halvorson, Orthopedic trauma surgeon and study investigator from Raleigh, NC says "I started using this only for periprosthetic fractures and have now used it when I need to increase stability and the construct stiffness of the fracture. I don't have to worry about screwing into the bone. The IlluminOss system augments poor bone, allowing for an increase in bone strength. Having the IlluminOss implant gives my patients the opportunity for early mobilization and weight bearing."
About IlluminOss Medical, Inc.
Headquartered in East Providence, RI, IlluminOss is a privately held, commercial-stage medical device company. The IlluminOss system is CE-marked and FDA-cleared for a variety of anatomical sites, with further indications pending. For additional information, including a complete list of indications, contraindications, warnings, precautions and risks, visit
www.illuminoss.com
.
For any press questions, please contact
Lisa Holt, at
[email protected]
.
SOURCE IlluminOss Medical
更多内容,请访问原始网站
文中所述内容并不反映新药情报库及其所属公司任何意见及观点,如有版权侵扰或错误之处,请及时联系我们,我们会在24小时内配合处理。
靶点
-药物
-生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。



