预约演示
New Publication Supports Clinical Effectiveness of Centinel Spine's Stand-Alone STALIF® M Integrated Interbody™ Portfolio
2022-11-30
临床结果临床研究
Study conducted by the Texas Back Institute (TBI), Plano, TX and published in the International Journal of Spine Surgery
The retrospective study supports utilizing
STALIF M
portfolio
implants as a stand-alone device
The STALIF technology platform has a proven clinical history of over 30 years and over 75,000 devices implanted
WEST CHESTER, Pa., Nov. 30, 2022 /PRNewswire/ -- Centinel Spine®, LLC, ("the Company") a leading global medical device company addressing cervical and lumbar spinal disease through anterior surgical access, today announced the recent publication of a peer-reviewed study of its
STALIF® M portfolio of products in the International Journal of Spine Surgery, titled: "Evaluation of Anterior Lumbar Interbody Fusion Performed Using a Stand-Alone, Integrated Fusion Cage." 1 The retrospective study, conducted by the Texas Back Institute (TBI), Plano, Texas, supports utilizing
STALIF M portfolio implants as a stand-alone device that produces good outcomes in patients treated for symptomatic disc degeneration while avoiding the use of posterior fixation and its complication risk and cost.
Continue Reading
Preview
来源: PRNewswire
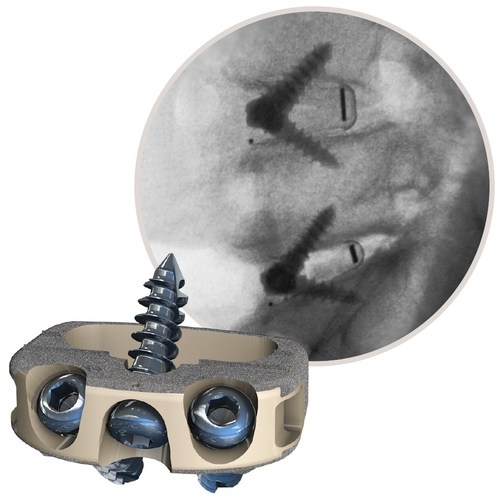
STALIF M-Ti
"The
STALIF M-Ti titanium-coated PEEK cage with integrated screws provides a stable construct, encouraging rapid fusion incorporation. The results of this study further support what I've seen clinically for many years, which is good outcomes from stand-alone ALIF for treating painful disc degeneration resistant to non-operative care," commented Scott L. Blumenthal, MD, TBI.
The
STALIF M portfolio of stand-alone integrated cage-screw implants were utilized in a consecutive series of 58 patients with a mean follow-up time of 30.6 months (minimum 24-month follow-up). All available patients' reported outcome scores demonstrated significant improvement (pJessica Shellock, MD, TBI. "Standalone ALIF is a very reasonable alternative to consider for patients that don't require an additional posterior surgery due to instability or severe neural compression and avoids the risks and cost associated with posterior instrumentation."
The
STALIF M implant technology has been engineered based on the
STALIF technology platform, which has a proven clinical history of over 30 years and over 75,000 devices implanted2.
"It has a long track record as an anatomic integrated ALIF implant that can be used as a stand-alone device. I like that the
STALIF M-Ti has an advanced titanium coating with a modulus of elasticity similar to bone, which minimizes subsidence. I have been extremely happy with its performance," said Richard D. Guyer, MD, TBI.
"The
STALIF M has been my 'go-to' standalone ALIF implant-of-choice for many years," commented Jack Zigler, MD, TBI. "It has a good range of sizes and lordotic angles, and has been consistently hassle-free in using the variable-angle screwdriver and self-tapping screws. After a tough decompression and mobilization, it's great to know that the implantation itself will be straightforward and will not introduce new problems at the end of the case," he concluded.
1 Richard D. Guyer, Jack E. Zigler, Scott L. Blumenthal, Jessica L. Shellock and Donna D. Ohnmeiss. Evaluation of Anterior Lumbar Interbody Fusion Performed Using a Stand-Alone, Integrated Fusion Cage. International Journal of Spine Surgery (Aug. 8, 2022). http://ijssurgery.com/content/early/2022/08/07/8354
2 Data on file
About Centinel Spine, LLC
Centinel Spine®, LLC is a leading global medical device company addressing cervical and lumbar spinal disease through anterior surgical access. The company offers a continuum of trusted, brand-name, motion-preserving and fusion solutions backed by over 30 years of clinical success—providing the most robust and clinically-proven technology platforms in the world for total disc replacement (pro
disc®) and Integrated Interbody™ fusion (
STALIF®).
Centinel Spine continues to advance its pioneering culture and corporate mission to become a catalyst of change in the spine industry and alter the way spine surgery is perceived. Centinel Spine remains the only company with comprehensive motion-preserving and fusion solutions for both cervical and lumbar anterior column reconstruction.
For more information, please visit the company's website at www.CentinelSpine.com or contact:
Centinel Spine
Varun Gandhi
Chief Financial Officer
900 Airport Road, Suite 3B
West Chester, PA 19380
Phone: 484-887-8871
Email: [email protected]
Media
Kyle Evans
ICR Westwicke
Phone: 646-677-1295
Email: [email protected]
SOURCE Centinel Spine, LLC
更多内容,请访问原始网站
文中所述内容并不反映新药情报库及其所属公司任何意见及观点,如有版权侵扰或错误之处,请及时联系我们,我们会在24小时内配合处理。
靶点
-药物
-生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。




