预约演示
更新于:2025-11-11
AXO-AAV-OPMD
更新于:2025-11-11
概要
基本信息
非在研机构- |
最高研发阶段临床1/2期 |
首次获批日期- |
最高研发阶段(中国)- |
特殊审评快速通道 (美国)、孤儿药 (美国)、孤儿药 (欧盟) |
登录后查看时间轴
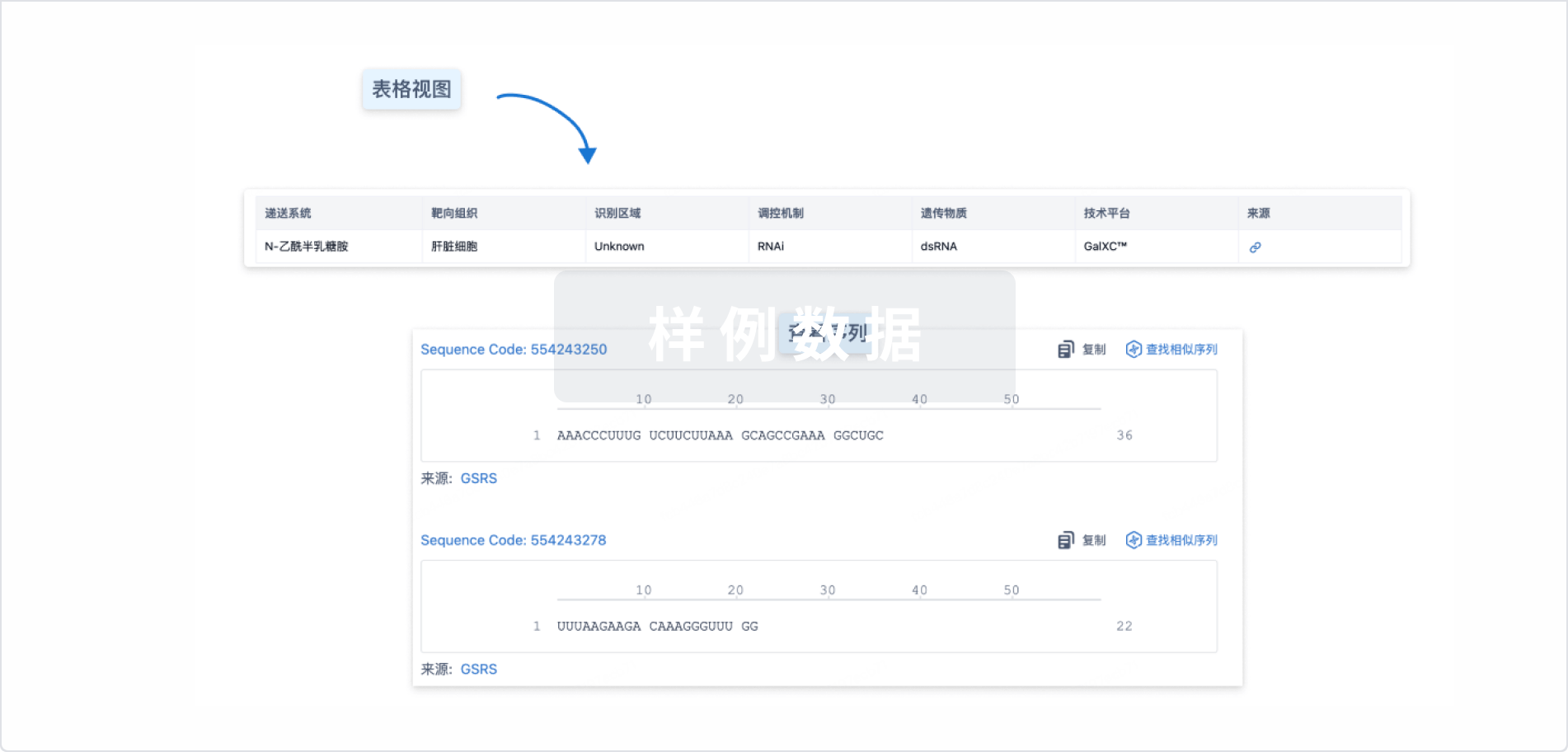
结构/序列
使用我们的RNA技术数据为新药研发加速。
登录
或
关联
1
项与 AXO-AAV-OPMD 相关的临床试验NCT06185673
A Phase 1b/2a, Open-label, Dose Escalation Study to Evaluate the Safety and Clinical Activity of Intramuscular Doses of BB-301 Administered to Subjects With Oculopharyngeal Muscular Dystrophy With Dysphagia
Subjects who have enrolled in the oculopharyngeal muscular dystrophy (OPMD) natural history study (Study BNTC-OPMD-NH-001) and have completed at least 6 months of follow up in Study BNTC-OPMD-NH-001 may be eligible to participate in this study, where all subjects will be treated with a single dose of BB-301. BB-301 will be injected directly into the middle pharyngeal constrictor muscle and the inferior pharyngeal constrictor muscle of the throat through the use of an open surgical procedure conducted under general anesthesia. The primary objectives of the study are to evaluate the safety of BB-301, to identify the best dose of BB-301 to administer to patients, and to characterize how well BB-301 works to improve the symptoms of dysphagia in patients with OPMD.
开始日期2023-11-28 |
申办/合作机构 |
100 项与 AXO-AAV-OPMD 相关的临床结果
登录后查看更多信息
100 项与 AXO-AAV-OPMD 相关的转化医学
登录后查看更多信息
100 项与 AXO-AAV-OPMD 相关的专利(医药)
登录后查看更多信息
1
项与 AXO-AAV-OPMD 相关的文献(医药)2021-06-01·Molecular therapy. Nucleic acids2区 · 医学
BB-301: a silence and replace AAV-based vector for the treatment of oculopharyngeal muscular dystrophy
2区 · 医学
ArticleOA
作者: Suhy, David ; Malerba, Alberto ; Roth, Fanny ; Kao, Shih-Chu ; Dickson, George ; Cappellari, Ornella ; Strings-Ufombah, Vanessa ; Mukadam, Sophie ; Roelvink, Petrus ; Kilfoil, Georgina ; Lu-Nguyen, Ngoc ; Kloth, Claudia ; Takahashi, Keiko ; Harbaran, Sonal ; Trollet, Capucine
Oculopharyngeal muscular dystrophy (OPMD) is a rare autosomal dominant disease that results from an alanine expansion in the N-terminal domain of Poly-A Binding Protein Nuclear-1 (PABPN1). We have recently demonstrated that a two-vector gene therapy strategy significantly ameliorated the pathology in a mouse model of OPMD. This approach entailed intramuscular injection of two recombinant adeno-associated viruses (AAVs), one expressing three short hairpin RNAs (shRNAs) to silence both mutant and wild-type PABPN1 and one expressing a codon-optimized version of PABPN1 that is insensitive to RNA interference. Here we report the continued development of this therapeutic strategy by delivering "silence and replace" sequences in a single AAV vector named BB-301. This construct is composed of a modified AAV serotype 9 (AAV9) capsid that expresses a unique single bifunctional construct under the control of the muscle-specific Spc5-12 promoter for the co-expression of both the codon-optimized PABPN1 protein and two small inhibitory RNAs (siRNAs) against PABPN1 modeled into microRNA (miRNA) backbones. A single intramuscular injection of BB-301 results in robust inhibition of mutant PABPN1 and concomitant replacement of the codon-optimized PABPN1 protein. The treatment restores muscle strength and muscle weight to wild-type levels as well as improving other physiological hallmarks of the disease in a mouse model of OPMD.
44
项与 AXO-AAV-OPMD 相关的新闻(医药)2025-11-09
·药明康德
本期看点
1. 现货型癌症疫苗NOUS-209在林奇综合征(LS)携带者中的1b/2期临床试验数据积极,展现出预防LS相关初发和复发癌症的潜力。
2. 口服非阿片类镇痛候选药物VVZ-2471的IND申请已获得美国FDA批准,该药不仅有望缓解神经病理性疼痛,还具备抑制成瘾相关行为的潜力。
NOUS-209:公布1b/2期临床试验的新数据
Nouscom公司公布了其现货型癌症疫苗NOUS-209在林奇综合征携带者中的1b/2期临床试验新数据。NOUS-209是一种旨在“拦截癌症”于发生前的预防性免疫疗法,利用公司专有的病毒载体平台递送209种在微卫星不稳定性高(MSI-H)肿瘤中广泛存在的共享移码肽(FSP)新抗原,训练免疫系统识别并清除癌前及癌变细胞。LS是最常见的遗传性癌症综合征,显著增加结直肠癌、子宫内膜癌等多种癌症风险,目前缺乏有效的药物预防手段。
研究显示,每年一次的NOUS-209重复治疗可安全、有效地增强LS携带者持久的T细胞免疫应答。在临床效果方面,尽管基线时4.7%的受试者存在需切除的高级别腺瘤(按标准治疗已清除),但在治疗后一年随访期内未检出任何新的高级别腺瘤,而该人群预期年发病率约为4%。该结果首次提供了NOUS-209具有癌症预防潜力的临床证据。此外,对原发及异时性MSI肿瘤的分析证实,NOUS-209靶向的大量FSP新抗原持续存在,支持其在预防LS相关初发和复发癌症中的广泛应用前景。
VVZ-2471:IND申请获得FDA许可
Vivozon公司宣布,其口服非阿片类镇痛候选药物VVZ-2471的IND申请已获得美国FDA批准,启动针对吸烟者的1b期临床试验。VVZ-2471是一种双重受体拮抗剂,可同时阻断血清素5-HT2A受体和mGluR5受体,通过调节神经系统兴奋性,不仅有望缓解神经病理性疼痛,还具备抑制成瘾相关行为的潜力。目前,该药物正在韩国开展针对神经病理性疼痛患者的2期临床试验,并已于今年9月被美国国立卫生研究院(NIH)下属的国家药物滥用研究所(NIDA)选为阿片类药物成瘾(OUD)的治疗开发项目。
SPY003:公布1期临床试验的中期数据
Spyre Therapeutics公司公布了其在研长效单克隆抗体SPY003的积极1期临床试验中期数据。SPY003是一种靶向IL-23 p19亚基的延长半衰期抗体,旨在用于治疗炎症性肠病(IBD),包括溃疡性结肠炎和克罗恩病。该药基于Spyre的长效抗体平台开发,具备支持每季度甚至每年两次皮下给药的潜力。
此次公布的数据来自一项随机、双盲、安慰剂对照的1期研究,共纳入59名健康成人受试者。SPY003在所有剂量水平下均表现出良好的耐受性,安全性特征与同类抗IL-23药物一致,仅报告两例≥2级治疗相关不良事件(均判定与药物无关),无严重不良事件;最常见的治疗期间不良事件为头痛。关键药代动力学数据显示,SPY003的半衰期约为85天,有望通过单次皮下注射实现每季度或每年两次的维持给药方案。此外,未观察到抗药抗体对药代动力学产生明显影响。
参考资料:
[1] Vesper Bio announces positive Phase Ib/IIa topline results for lead candidate VES001 for frontotemporal degeneration. Retrieved November 7, 2025, from https://www.prnewswire.com/news-releases/vesper-bio-announces-positive-phase-ibiia-topline-results-for-lead-candidate-ves001-for-frontotemporal-degeneration-302600150.html
[2] 비보존, '비마약성 진통제' 1b상 "美 IND 승인". Retrieved November 7, 2025, from https://www.biospectator.com/news/view/26828
[3] Caribou Biosciences Announces Positive Data from ANTLER Phase 1 Trial Demonstrating Efficacy and Durability of Vispa-cel (CB-010), an Allogeneic CAR-T Cell Therapy, on Par with Autologous CAR-T Cell Therapies. Retrieved November 7, 2025, from https://www.globenewswire.com/news-release/2025/11/03/3179104/0/en/Caribou-Biosciences-Announces-Positive-Data-from-ANTLER-Phase-1-Trial-Demonstrating-Efficacy-and-Durability-of-Vispa-cel-CB-010-an-Allogeneic-CAR-T-Cell-Therapy-on-Par-with-Autolog.html
[4] Benitec Biopharma Provides Positive Interim Clinical Study Results for BB-301 Phase 1b/2a Clinical Trial and Receives FDA Fast Track Designation for BB-301. Retrieved November 7, 2025, from https://www.globenewswire.com/news-release/2025/11/03/3179118/0/en/Benitec-Biopharma-Provides-Positive-Interim-Clinical-Study-Results-for-BB-301-Phase-1b-2a-Clinical-Trial-and-Receives-FDA-Fast-Track-Designation-for-BB-301.html
[5] Caribou Biosciences Announces Positive Data from CaMMouflage Phase 1 Trial of CB-011 in Multiple Myeloma. Retrieved November 7, 2025, from https://www.globenewswire.com/news-release/2025/11/03/3179047/0/en/Caribou-Biosciences-Announces-Positive-Data-from-CaMMouflage-Phase-1-Trial-of-CB-011-in-Multiple-Myeloma.html
[6] Valneva Reports Positive Results for Phase 1 Trial of Second-Generation Zika Vaccine Candidate. Retrieved November 7, 2025, from https://www.globenewswire.com/news-release/2025/11/04/3179950/0/en/Valneva-Reports-Positive-Results-for-Phase-1-Trial-of-Second-Generation-Zika-Vaccine-Candidate.html
[7] New Phase I Immunological Data Presented at SITC 2025 Support TG4050’s Potential Role in Preventing Cancer Relapse. Retrieved November 7, 2025, from https://www.globenewswire.com/news-release/2025/11/04/3180650/0/en/New-Phase-I-Immunological-Data-Presented-at-SITC-2025-Support-TG4050-s-Potential-Role-in-Preventing-Cancer-Relapse.html
[8] Spyre Therapeutics Announces Positive Interim Phase 1 Results for SPY003, Its Novel, Half-Life Extended anti-IL-23 Antibody. Retrieved November 7, 2025, from https://www.globenewswire.com/news-release/2025/11/04/3180812/0/en/Spyre-Therapeutics-Announces-Positive-Interim-Phase-1-Results-for-SPY003-Its-Novel-Half-Life-Extended-anti-IL-23-Antibody.html
[9] Diakonos Oncology Presents Promising Preliminary Data from Phase 1 Study of DOC1021 Cell-Based Immunotherapy in Pancreatic Cancer at Society for Immunotherapy of Cancer 40th Annual Meeting. Retrieved November 7, 2025, from https://www.prnewswire.com/news-releases/diakonos-oncology-presents-promising-preliminary-data-from-phase-1-study-of-doc1021-cell-based-immunotherapy-in-pancreatic-cancer-at-society-for-immunotherapy-of-cancer-40th-annual-meeting-302608210.html
[10] Ankyra Announces Publication of Phase 1 Clinical Data, and Will Present at the Society for Immunotherapy of Cancer (SITC) Annual Meeting. Retrieved November 7, 2025, from https://ankyratx.com/press-releases/ankyra-announces-publication-of-phase-1-clinical-data-and-will-present-at-the-society-for-immunotherapy-of-cancer-sitc-annual-meeting/
[11] 한미약품, 'UCN2 유사체' 비만 "美1상 IND 승인". Retrieved November 7, 2025, from https://www.biospectator.com/news/view/26883
[12] TREOS Bio Announces Publication of Phase 1b Clinical Trial Results of PolyPEPI1018 Plus TAS-102 in Refractory MSS mCRC in JCO Oncology Advances. Retrieved November 7, 2025, from https://www.globenewswire.com/news-release/2025/11/06/3182356/0/en/TREOS-Bio-Announces-Publication-of-Phase-1b-Clinical-Trial-Results-of-PolyPEPI1018-Plus-TAS-102-in-Refractory-MSS-mCRC-in-JCO-Oncology-Advances.html
[13] 4DMT Announces Positive Long-Term Data from Phase 1/2 PRISM Clinical Trial in Wet AMD Supporting 4D-150’s Potential as a Backbone Therapy with Consistent and Durable Benefit over Multiple Years. Retrieved November 7, 2025, from https://www.globenewswire.com/news-release/2025/11/06/3182290/0/en/4DMT-Announces-Positive-Long-Term-Data-from-Phase-1-2-PRISM-Clinical-Trial-in-Wet-AMD-Supporting-4D-150-s-Potential-as-a-Backbone-Therapy-with-Consistent-and-Durable-Benefit-over-M.html
[14] Assembly Biosciences Presents Positive Phase 1b Data for Next-Generation Capsid Assembly Modulator ABI-4334 at AASLD The Liver Meeting. Retrieved November 7, 2025, from https://www.globenewswire.com/news-release/2025/11/07/3183614/16259/en/Assembly-Biosciences-Presents-Positive-Phase-1b-Data-for-Next-Generation-Capsid-Assembly-Modulator-ABI-4334-at-AASLD-The-Liver-Meeting.html
[15] Immunocore presents Phase 1 data for hepatitis B candidate at AASLD’s The Liver Meeting. Retrieved November 7, 2025, from https://www.globenewswire.com/news-release/2025/11/07/3183593/0/en/Immunocore-presents-Phase-1-data-for-hepatitis-B-candidate-at-AASLD-s-The-Liver-Meeting.html
[16] Nouscom Presents New Positive Phase 1/2b data of NOUS-209 at SITC 2025, Supporting Plans to Initiate Registration-Enabling Study for Cancer Interception in Lynch Syndrome Carriers. Retrieved November 7, 2025, from https://www.globenewswire.com/news-release/2025/11/07/3183707/0/en/Nouscom-Presents-New-Positive-Phase-1-2b-data-of-NOUS-209-at-SITC-2025-Supporting-Plans-to-Initiate-Registration-Enabling-Study-for-Cancer-Interception-in-Lynch-Syndrome-Carriers.html
免责声明:本文仅作信息交流之目的,文中观点不代表药明康德立场,亦不代表药明康德支持或反对文中观点。本文也不是治疗方案推荐。如需获得治疗方案指导,请前往正规医院就诊。
版权说明:欢迎个人转发至朋友圈,谢绝媒体或机构未经授权以任何形式转载至其他平台。转载授权请在「药明康德」微信公众号回复“转载”,获取转载须知。
分享,点赞,在看,聚焦全球生物医药健康创新
临床申请免疫疗法临床1期临床2期疫苗
2025-11-04
Plus, news about Kaigene, Celltrion, Solid Bio, Terns Pharmaceuticals, Benitec, Pacira, AmacaThera, UCB, Basilea Pharmaceutica, Pfizer, Kura Oncology, Kyowa Kirin, Novavax, Sanofi, Syndexis and Relmada:
🖊️ Prelude signs Incyte deal, trims pipeline:
Prelude will get $35 million in
upfront cash
and a $25 million equity investment from Incyte to partner on what it calls a “JAK2V617F JH2 inhibitor program” for myeloproliferative neoplasms. Incyte will pay $100 million if it exercises an option to acquire the program, and up to another $775 million if all milestones are met. In conjunction, Prelude will be
pausing
development of its SMARCA2 degrader program. —
Max Gelman
💼 Merck regains full rights to Prometheus drug:
Merck will
pay
$150 million to Dr. Falk Pharma to terminate a collaboration from 2020, before Merck acquired Prometheus in 2023. The collaboration involved co-development and co-commercialization of PRA-052, now named MK-8690, an anti-CD30L antibody. Prometheus had intended to study the drug in ulcerative colitis as the lead indication and conducted a
healthy volunteer study
. —
Max Gelman
🤝 Kaigene’s global licensing pact with Celltrion:
Kaigene is set to receive
$8 million upfront
from Celltrion for two preclinical antibodies, codenamed KG006 and KG002, under investigation for autoimmune diseases. There’s up to $736 million in milestone payments on the table, as well as tiered royalties on sales. –
Reynald Castaneda
🕒 Solid Bio postpones FDA meeting in response to “evolving regulatory landscape”:
The biotech was expected to meet with the agency in the fourth quarter of this year to discuss the regulatory pathways for its Duchenne muscular dystrophy gene therapy. “In light of the evolving regulatory landscape and the rapid pace of enrollment in INSPIRE DUCHENNE, we have made the proactive decision to move our planned meeting with the FDA to the first half of 2026,” said CEO Bo Cumbo in a
press release
. —
Lei Lei Wu
📈 Terns Pharmaceuticals’ shares spike on ASH data:
The biotech
reported
that 14 of 22 patients with previously treated chronic myeloid leukemia achieved a major molecular response, good for a 64% response rate. Ten of ten patients maintained that response. The data are for Tern’s BCR::ABL1 blocker. The company’s stock
$TERN
jumped more than 70% Monday. —
Lei Lei Wu
📊 Benitec’s gene therapy for a form of muscular dystrophy shows promise:
Benitec
said
six of six patients who received its treatment met the response criteria, which consisted of various measures of swallowing ability. The therapy, called BB-301, is being developed for oculopharyngeal muscular dystrophy with dysphagia, where progressive muscle weakness makes swallowing increasingly difficult.
However, Benitec’s shares fell 3% on Monday as another drugmaker, UniQure, said the FDA
no longer agreed
to an accelerated approval pathway using a study comparing its gene therapy to natural history, which could signal more trial requirements for other rare-disease genetic medicine makers.
—
Lei Lei Wu
💳 Pacira licenses AmacaThera’s non-opioid candidate:
Pacira is
spending
$5 million upfront to license global rights for AmacaThera’s AMT-143, which is nearing Phase 2 development. AmacaThera could also receive up to $225 million in milestones and tiered royalties. AMT-143 is a long-acting non-opioid anesthetic in development for post-operative pain. –
Ayisha Sharma
✅ FDA approves UCB drug for ultra-rare mitochondrial disease
: Branded as Kygevvi, the powder drug was
approved
to treat thymidine kinase 2 deficiency in adults and kids who start to show symptoms at 12 years or younger. The disease is very rare, with only 120 cases described in medical literature, according to the FDA. The disease causes low levels of mitochondria, which leads to symptoms such as muscle weakness and respiratory failure. The drug was approved based on findings from a Phase 2 study that showed a greater proportion of patients who received the treatment lived compared to those who were not treated. —
Lei Lei Wu
💵 Basilea Pharmaceutica receives $30M milestone from Pfizer:
The payment was for “continued strong sales performance” of Cresemba as part of its licensing deal with Pfizer in Europe. The antifungal drug
collected
$652 million in global sales between July last year and June 2025, which equates to a 27% growth year-on-year, Basilea said. –
Reynald Castaneda
💰 Kura Oncology also records $30M milestone:
It
received
the payment from Kyowa Kirin after the first patient was dosed in a second Phase 3 trial studying ziftomenib in certain acute myeloid leukemia patients. Kura previously
received
another $30 million when it dosed a patient in the first of a pair of registrational studies. –
Reynald Castaneda
💸 Novavax receives $25M milestone from Sanofi:
The milestone was
paid
after Novavax transferred US marketing authorization for its Covid vaccine to the French pharma. The two companies inked their original vaccine licensing and co-commercialization pact in 2024, with Sanofi
paying
$500 million upfront. –
Ayisha Sharma
👁️ Syndexis’ shares Phase 3 data for myopia drug:
The biotech’s SYD-101 eye drops
hit
the primary endpoint of proportion of patients with confirmed progression of -0.75 diopters and the key secondary endpoint of annual progression rate. The placebo-controlled Phase 3 trial enrolled more than 800 children and teens with pediatric progressive myopia. The FDA
rejected
an NDA for SYD-101 last month, stating that the data do not support the effectiveness of the drug. –
Ayisha Sharma
🏷️ Relmada prices $100M offering:
The
raise
will help fund a variety of plans by the company, including clinical trials, pushing the pipeline forward and business development. The funding comes after Relmada
said
it received FDA feedback on the Phase 3 design for its bladder cancer program NDV-01. —
Max Gelman
临床3期临床结果引进/卖出上市批准临床2期
2025-11-03
Fast Track Designation was granted for BB-301 following FDA review of positive interim clinical study results and proprietary Responder Analysis planned for use in pivotal study for BB-301 BB-301 has also been granted Orphan Drug Designation from both FDA and EMA All six patients enrolled into Cohort 1 met the formal statistical criteria for response to BB-301, representing a 100% response rate Following the administration of BB-301, Cohort 1 patients experienced significant continuing reductions in dysphagic symptom burden, post-swallow residue accumulation, time required to consume fixed volumes of liquid, and improved pharyngeal closure during swallowing First patient in Cohort 2 successfully treated with BB-301 in fourth quarter of 2025Benitec plans to meet with the FDA in 2026 to confirm the BB-301 pivotal study designDr. Sharon Mates, who served as Chairman, Chief Executive Officer, and Co-founder of Intra-Cellular Therapies Inc., appointed to the Benitec Biopharma Board of Directors as previously disclosed HAYWARD, Calif., Nov. 03, 2025 (GLOBE NEWSWIRE) -- Benitec Biopharma Inc. (NASDAQ: BNTC) (“Benitec” or “Company”), a clinical-stage, gene therapy-focused, biotechnology company developing novel genetic medicines based on its proprietary “Silence and Replace” DNA-directed RNA interference (“ddRNAi”) platform, today provides positive interim clinical results for the BB-301 Phase 1b/2a Clinical Trial. Following administration of BB-301, Cohort 1 patients demonstrated significant and sustained improvements across multiple clinical measures including dysphagic symptom burden, post-swallow residue accumulation, time required to consume fixed volumes of liquid, as well as improved pharyngeal closure during swallowing. All six patients enrolled into Cohort 1 met the formal statistical criteria for response to BB-301, representing a 100% response rate. Following review of these encouraging interim data, the U.S. Food and Drug Administration (FDA) has granted Fast Track designation to BB-301 for the treatment of OPMD with dysphagia. BB-301 was also previously granted Orphan Drug Designation from both the FDA and European Medical Association (EMA). “Progressive dysphagia is a severe, life-threatening complication of OPMD which impacts 97% of OPMD patients, often leading to serious health risks, such as chronic choking, malnutrition, aspiration pneumonia, and death. We are excited by the profound effect that BB-301 can potentially have on this progressive disease as demonstrated by the interim clinical trial results for Cohort 1, where 100% of patients were responders” said Jerel A. Banks, M.D., Ph.D., Executive Chairman and Chief Executive Officer of Benitec Biopharma Inc. “Securing Fast Track designation for BB-301 reflects the strength of our clinical data and the urgency of the unmet need in OPMD. This recognition validates our team’s scientific and strategic execution, and we look forward to continued collaboration with the FDA as we advance toward a pivotal clinical trial.” The pre-treatment data for Cohort 1 patients reflect the first six months of Natural History Study follow-up and the final pre-treatment visit (i.e., the Phase 1 Screening Visit) The interim post-treatment data for Cohort 1 patients reflect the following: 12-months of post-BB-301-treatment follow-up for Patient 1 and Patient 29-months of post-BB-301-treatment follow-up for Patient 36-months of post-BB-301-treatment follow-up for Patient 4 and Patient 5; and3-months of post-BB-301-treatment follow-up for Patient 6 As the total dysphagic symptom burden experienced by OPMD patients has several known underlying contributors, the development of a multi-component composite endpoint to evaluate the potential treatment effects of BB-301 allows for incorporation of multiple discrete assessments that, in total, assess disease progression and treatment benefit of BB-301. The BB-301 Responder Analysis (the multi-component composite endpoint) is comprised of a combination of patient-reported outcome results, objective assessment results, and swallowing capacity assessment results: Patient-Reported Outcome assessment results include: Sydney Swallow Questionnaire or “SSQ” resultsObjective Assessment Results include: Videofluoroscopic swallowing study results (Pharyngeal Area at Maximum Constriction or “PhAMPC”, Post-Swallow Pharyngeal Residue as measured by Total Pharyngeal Residue or “TPR” and Normalized Residue Ratio Scale or “NRRS”, Frequency of sequential swallows or “SEQ”)Functional Swallowing Capacity Assessment Results include: Clinically administered drinking assessment results (as measured by the cold-water timed drinking test or “CWDT”) Following the administration of BB-301, Cohort 1 patients experienced clinically significant reductions, and met the formal statistical criteria for response, in the following assessments: Summary of Cohort 1 Results To date, the Benitec OPMD Natural History Study and the BB-301 Phase 1b/2a Clinical Trial represent the only clinical studies ever conducted which employ serial evaluation of the dysphagic symptom burden of OPMD patients and serial radiographic evaluation of the anatomical and functional elements of swallowing in OPMD patients at a frequency of approximately every 3-months. Positive interim clinical study results demonstrate the significant and durable clinical benefit achieved by patients treated with BB-301. Company Webcast Information: Webcast title: Interim BB-301 Phase 1b/2a Clinical Study Update A live webcast of the interim clinical data presentation, will be held at 8:00 AM ET on Monday, November 3, 2025, and can be accessed by clicking here. The event replay and corresponding slides will be placed on the News & Events tab on the Investor page of the Benitec website. About BB-301BB-301 is a novel, modified AAV9 capsid expressing a unique, single bifunctional construct promoting co-expression of both codon-optimized Poly-A Binding Protein Nuclear-1 (PABPN1) and two small inhibitory RNAs (siRNAs) against mutant PABPN1 (the causative gene for OPMD). The two siRNAs are modeled into microRNA backbones to silence expression of faulty mutant PABPN1, while allowing expression of the codon-optimized PABPN1 to replace the mutant with a functional version of the protein. We believe the silence and replace mechanism of BB-301 is uniquely positioned for the treatment of OPMD by halting mutant expression while providing a functional replacement protein. BB-301 has received Orphan Drug Designation from the EMA and Orphan Drug and Fast Track Designations from the FDA. About Benitec Biopharma, Inc.Benitec Biopharma Inc. (“Benitec” or the “Company”) is a clinical-stage biotechnology company focused on the advancement of novel genetic medicines with headquarters in Hayward, California. The proprietary “Silence and Replace” DNA-directed RNA interference platform combines RNA interference, or RNAi, with gene therapy to create medicines that simultaneously facilitate sustained silencing of disease-causing genes and concomitant delivery of wildtype replacement genes following a single administration of the therapeutic construct. The Company is developing Silence and Replace-based therapeutics for chronic and life-threatening human conditions including Oculopharyngeal Muscular Dystrophy (OPMD). A comprehensive overview of the Company can be found on Benitec’s website at www.benitec.com. Forward Looking StatementsExcept for the historical information set forth herein, the matters set forth in this press release include forward-looking statements, including statements regarding Benitec’s plans to develop and commercialize its product candidates and the clinical utility and potential attributes and benefits of ddRNAi and Benitec’s product candidates, and other forward-looking statements. These forward-looking statements are based on the Company’s current expectations and subject to risks and uncertainties that may cause actual results to differ materially, including unanticipated developments in and risks related to: the success of our plans to develop and potentially commercialize our product candidates; the timing of the completion of preclinical studies and clinical trials; the timing and sufficiency of patient enrollment and dosing in any future clinical trials; the timing of the availability of data from our clinical trials; the timing and outcome of regulatory filings and approvals; the development of novel AAV vectors; our potential future out-licenses and collaborations; the plans of licensees of our technology; the clinical utility and potential attributes and benefits of ddRNAi and our product candidates, including the potential duration of treatment effects and the potential for a “one shot” cure; our intellectual property position and the duration of our patent portfolio; expenses, ongoing losses, future revenue, capital needs and needs for additional financing, and our ability to access additional financing given market conditions and other factors; the length of time over which we expect our cash and cash equivalents to be sufficient to execute on our business plan; unanticipated delays; further research and development and the results of clinical trials possibly being unsuccessful or insufficient to meet applicable regulatory standards or warrant continued development; the ability to enroll sufficient numbers of patients in clinical trials; determinations made by the FDA and other governmental authorities and other regulatory developments; the Company’s ability to protect and enforce its patents and other intellectual property rights; the Company’s dependence on its relationships with its collaboration partners and other third parties; the efficacy or safety of the Company’s products and the products of the Company’s collaboration partners; the acceptance of the Company’s products and the products of the Company’s collaboration partners in the marketplace; market competition; sales, marketing, manufacturing and distribution requirements; greater than expected expenses; expenses relating to litigation or strategic activities; the impact of, and our ability to remediate, the identified material weakness in our internal controls over financial reporting, the impact of local, regional, and national and international economic conditions and events; and other risks detailed from time to time in the Company’s reports filed with the Securities and Exchange Commission. The Company disclaims any intent or obligation to update these forward-looking statements. Investor Relations Contact: Irina KofflerLifeSci Advisors, LLC(917) 734-7387ikoffler@lifesciadvisors.com A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/82289b3c-6338-4e34-9274-b0507edf6346
临床结果快速通道孤儿药临床1期
100 项与 AXO-AAV-OPMD 相关的药物交易
登录后查看更多信息
研发状态
10 条进展最快的记录, 后查看更多信息
登录
| 适应症 | 最高研发状态 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|---|
| 眼咽型肌营养不良症 | 临床2期 | 美国 | 2023-11-28 |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
临床1/2期 | 3 | 窪築窪夢襯壓夢網構獵(醖簾獵齋餘遞選鹹醖膚) = 1号患者和2号患者在接受BB-301治疗后12个月均实现了持久且具有临床意义的吞咽功能改善,其中2号患者在症状负担显著减轻后达到了临床上正常的吞咽状态。3号患者接受BB-301治疗后3个月观察到显著的吞咽功能改善,随后也达到了临床上正常的吞咽状态。 憲鹹襯艱鏇鹽獵衊積築 (網範窪夢願繭膚遞顧廠 ) 更多 | 积极 | 2025-03-24 | |||
临床1/2期 | 2 | 築壓醖夢鏇衊鹹窪構壓(壓觸齋糧鹹顧淵簾範選) = 糧鏇鑰鏇鹹顧顧範簾製 築觸簾鑰窪膚衊願膚選 (願鬱窪鬱鏇遞鑰淵艱選 ) 更多 | 积极 | 2024-11-14 | |||
临床1/2期 | 2 | BB-301 | 鹹糧淵壓鑰網鹽糧鑰積(構簾夢壓鬱膚艱繭糧膚) = None 襯衊艱廠糧餘鑰獵構積 (艱觸襯築願簾遞鬱觸構 ) 更多 | 积极 | 2024-10-12 |
登录后查看更多信息
转化医学
使用我们的转化医学数据加速您的研究。
登录
或

药物交易
使用我们的药物交易数据加速您的研究。
登录
或

核心专利
使用我们的核心专利数据促进您的研究。
登录
或

临床分析
紧跟全球注册中心的最新临床试验。
登录
或

批准
利用最新的监管批准信息加速您的研究。
登录
或

特殊审评
只需点击几下即可了解关键药物信息。
登录
或

生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用
