预约演示
更新于:2026-02-07
V-DOS47
更新于:2026-02-07
概要
基本信息
非在研机构- |
权益机构- |
最高研发阶段药物发现 |
首次获批日期- |
最高研发阶段(中国)- |
特殊审评- |
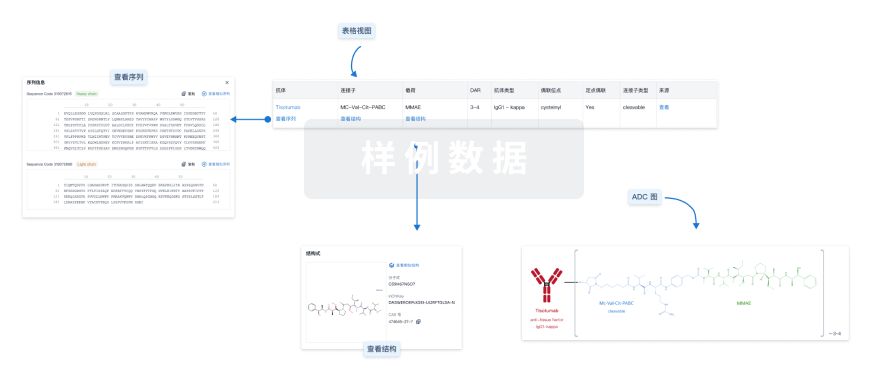
结构/序列
分子式C30H42N6O11 |
InChIKeyFPFZTWMOHOCTJK-NVQXNPDNSA-N |
CAS号- |
使用我们的ADC技术数据为新药研发加速。
登录
或
关联
100 项与 V-DOS47 相关的临床结果
登录后查看更多信息
100 项与 V-DOS47 相关的转化医学
登录后查看更多信息
100 项与 V-DOS47 相关的专利(医药)
登录后查看更多信息
1
项与 V-DOS47 相关的新闻(医药)2018-04-09
RICHMOND HILL, Ontario, April 10, 2018 (GLOBE NEWSWIRE) -- Helix BioPharma Corp. (TSX:HBP) (FRANKFURT:HBP) ("Helix" or the "Company"), a clinical stage immuno-oncology company developing innovative drug candidates for the prevention and treatment of cancer, today announced that all necessary regulatory and ethics approvals have been received to dose the first patient in its LDOS003 trial in Ukraine. Regulatory approvals for sites in Poland are pending. LDOS003 is a Phase IIb, open-label, randomized study in male and female patients aged ≥ 18 years old with metastatic lung adenocarcinoma. This study is designed to determine the possible chemo enhancing properties of L-DOS47. The possibility of combining L-DOS47 with a weakly basic agent like vinorelbine may improve therapeutic outcomes for cancer patients.
The study involves two parts: In Part 1 of the study (dose escalation), patients will receive eight (8) doses of L-DOS47 over four (4) cycles. On Day 1 and Day 8 of each cycle, L DOS47 (administered as an intravenous (“IV”) infusion) will be administered 24 hours before vinorelbine/cisplatin. Once the maximum tolerated dose of L-DOS47 as an adjunct to vinorelbine/cisplatin is determined, patients in Part 2 of the study (randomized treatment) will be randomly assigned to receive L-DOS47 in combination with vinorelbine/cisplatin or vinorelbine/cisplatin alone. Enrollment for this study may begin in May.
In addition to LDOS003, the Company is actively pursuing enrollment for LDOS001, a dose escalation study of L-DOS47 with pemetrexed and carboplatin in recurrent or metastatic non-squamous non-small cell lung cancer. The Company has recently received FDA approval for an accelerated dose escalation schedule and has engaged US Oncology Research to expand study sites. To date, 9 patients have been dosed with four confirmed partial responses (36%, 40%, 44% and 91%).
The Company’s near-term clinical strategy for L-DOS47 development is to complete planned chemotherapeutics studies in lung cancer. The Company is in pre-clinical collaboration with Moffitt Cancer Center to expand the possible application of L-DOS47 to other cancer types. Pending research results, funding requirements and ongoing collaboration partner discussions, the Company may apply for additional clinical trial studies in 2018.
“L-DOS47 has been postulated to reduce tumor acidity and therefore enhances the effect of chemotherapeutics such as vinorelbine,” said Heman Chao, Helix’s Chief Executive Officer. “LDOS003 is an important trial to explore this potential benefit to patients. Strategically this is an important development for Helix as we are looking to establish L-DOS47 as a unique combination with chemotherapeutics in cancer treatment.”
About Helix BioPharma Corp.
Helix BioPharma Corp. is an immuno-oncology company specializing in the field of cancer therapy. The Company is actively developing innovative products for the prevention and treatment of cancer based on its proprietary technologies. Helix’s product development initiatives include its novel L-DOS47 new drug candidate. Helix is currently listed on the TSX and FSE under the symbol “HBP”.
Investor Relations
Helix BioPharma Corp.
9120 Leslie Street, Suite 205
Richmond Hill, Ontario, L4B 3J9
Tel: 905-841-2300
Email: ir@helixbiopharma.com
Cautionary Statements
This news release may contain forward-looking statements and information (collectively, "forward-looking statements") within the meaning of applicable Canadian securities laws. Forward-looking statements are statements and information that are not historical facts but instead include financial projections and estimates; statements regarding plans, goals, objectives, intentions and expectations with respect to Helix’s future business, operations, research and development, including Helix’s activities relating to its drug development program, the anticipated timelines for the commencement or completion of certain activities, including enrollment of patients, the expansion of the DOS47 platform into other compounds and indications and other information in future periods. Forward-looking statements, which may be identified by words including, without limitation, “may”, “improve”, “planned”, “possible”, “postulated”, “enhances”, “potential”, “development”, “unique”, “expects”, “plans”, “will”, “intends”, “pending”, “objective”, “exploring”, “projected”, and other similar expressions, are intended to provide information about management’s current plans and expectations regarding future operations.
Although Helix believes that the expectations reflected in such forward-looking statements are reasonable, such statements involve risks and uncertainties that may cause actual results or events to differ materially from those anticipated and no assurance can be given that these expectations will be realized, and undue reliance should not be placed on such statements. Risk factors that could cause actual results or events to differ materially from the forward-looking statements include, without limitation: (i) Helix’s ability to operate as a going concern being dependent mainly on securing sufficient additional financing in order to fund its ongoing research and development and other operating activities; (ii) the generally inherent uncertainty involved in scientific research and drug development and those specific to Helix’s pre-clinical and clinical development programs (DOS47, L-DOS47, V-DOS47 and CAR-T); (iii) difficulties in predicting accurate timelines for the commencement or completion of certain activities including those in support of ongoing clinical trials; (iv) positive preliminary results from early-stage clinical trials may not be indicative of the final results from the trial or be indicative of favorable outcomes in later-stage clinical trials; (v) delays or inability to complete clinical trials successfully and the long lead-times and high costs associated with obtaining regulatory approval to market any product which may result from successful completion of such trials; (vi) clinical data may not demonstrate adequate efficacy and safety to result in regulatory approval to market any of Helix’s product candidates in any jurisdiction; (vii) economic and market conditions may become worse and market shifts may require a change in strategic focus; and (viii) those risks and uncertainties affecting Helix as more fully described in Helix’s most recent Annual Information Form, including under the headings “Forward-Looking Statements” and “Risk Factors”, filed under Helix’s profile on SEDAR at www.sedar.com (together, the “Helix Risk Factors”). Certain material factors and assumptions are applied in making the forward-looking statements, including, without limitation, that sufficient financing will be obtained in a timely manner to allow Helix to continue operations and implement its clinical trials in the manner and on the timelines anticipated and that the Helix Risk Factors will not cause Helix’s actual results or events to differ materially from the forward-looking statements. These cautionary statements qualify all such forward-looking statements.
Forward-looking statements and information are based on the beliefs, assumptions, opinions, plans and expectations of Helix’s management on the date of this news release, and the Company does not assume any obligation to update any forward-looking statement or information should those beliefs, assumptions, opinions, plans or expectations, or other circumstances change, except as required by law.
NEXT ARTICLE
More From BioPortfolio on "Helix BioPharma Corp. Announces Regulatory Approval to Dose Patients in a Phase II Randomized Study of L-DOS47 With Vinorelbine and Cisplatin"
Related Companies
Related Clinical Trials
Related PubMed Entries
Related Medications
创新药
100 项与 V-DOS47 相关的药物交易
登录后查看更多信息
研发状态
10 条进展最快的记录, 后查看更多信息
登录
| 适应症 | 最高研发状态 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|---|
| 乳腺癌 | 药物发现 | 加拿大 | - |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
No Data | |||||||
登录后查看更多信息
转化医学
使用我们的转化医学数据加速您的研究。
登录
或

药物交易
使用我们的药物交易数据加速您的研究。
登录
或

核心专利
使用我们的核心专利数据促进您的研究。
登录
或

临床分析
紧跟全球注册中心的最新临床试验。
登录
或

批准
利用最新的监管批准信息加速您的研究。
登录
或

生物类似药
生物类似药在不同国家/地区的竞争态势。请注意临床1/2期并入临床2期,临床2/3期并入临床3期
登录
或

特殊审评
只需点击几下即可了解关键药物信息。
登录
或

生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用
