预约演示
更新于:2026-01-31
BP-1003
更新于:2026-01-31
概要
基本信息
非在研机构- |
权益机构- |
最高研发阶段临床申请 |
首次获批日期- |
最高研发阶段(中国)- |
特殊审评- |
登录后查看时间轴
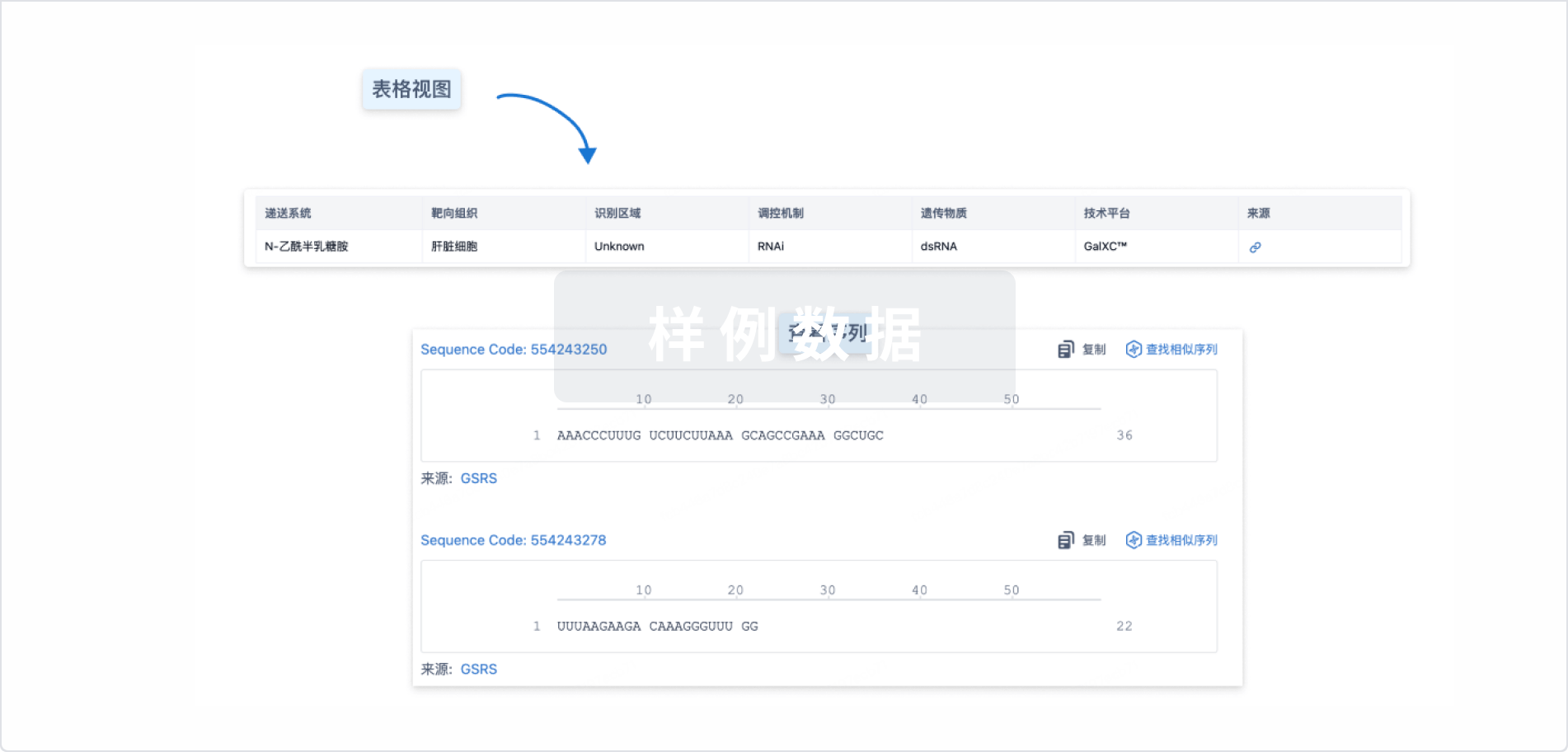
结构/序列
使用我们的RNA技术数据为新药研发加速。
登录
或
关联
100 项与 BP-1003 相关的临床结果
登录后查看更多信息
100 项与 BP-1003 相关的转化医学
登录后查看更多信息
100 项与 BP-1003 相关的专利(医药)
登录后查看更多信息
2
项与 BP-1003 相关的文献(医药)2004-08-01·Archives of dermatological research3区 · 医学
?-Lipoic acid-based PPAR? agonists for treating inflammatory skin diseases
3区 · 医学
Article
作者: Meenakshi S. Venkatraman ; Charles N. Ellis ; Josef Meingassner ; Mitchell A. Avery ; Theodore W. Kurtz ; Harrihar A. Pershadsingh ; James Varani ; Amar Chittiboyina ; Stephen C. Benson ; Christopher I. Ho
Novel thiazolidinedione derivatives of the potent antioxidant, alpha-lipoic (thioctic, 1,2-dithiolane) acid, were prepared. The prototype N-(2-[4-[2,4-dioxo(1,3-thiazolidin-5-yl)methyl]phenoxy]ethyl)-5-(1,2-dithiolan-3-yl)- N-methylpentanamide (designated BP-1003), and dithioester derivatives thereof were shown to be potent activators of peroxisome proliferator-activated receptor gamma (PPARgamma) (EC(50) range 15-101 nM) and modest activators of PPARalpha (EC(50) 5 microM). Both the relatively hydrophobic dithiolane prototype, BP-1003, and its water-soluble dithioglycinate derivative, BP-1017, were shown to inhibit the proliferation of human keratinocytes and suppress the production of interleukin-2 by human peripheral lymphocytes to a greater extent than the antidiabetic thiazolidinedione, rosiglitazone. Both oral and topical administration of BP-1017 showed significant antiinflammatory effects in the oxazolone-sensitized mouse model of allergic contact dermatitis (ACD). These findings suggest that water-soluble lipoic acid-based thiazolidinediones may be efficacious as oral and topical agents for treating inflammatory skin conditions such as contact dermatitis, atopic dermatitis, and psoriasis.
Biomedicines
BP1003 Decreases STAT3 Expression and Its Pro-Tumorigenic Functions in Solid Tumors and the Tumor Microenvironment
Article
作者: Augustine, Jithesh Jose ; Gagliardi, Maria ; Ashizawa, Ana Tari ; Hooper, D. Craig ; Kean, Rhonda ; Roberts, Michael ; Fleming, Jason ; Dai, Bingbing
Overexpression and aberrant activation of signal transducer and activator of transcription 3 (STAT3) contribute to tumorigenesis, drug resistance, and tumor-immune evasion, making it a potential cancer therapeutic target. BP1003 is a neutral liposome incorporated with a nuclease-resistant P-ethoxy antisense oligodeoxynucleotide (ASO) targeting the STAT3 mRNA. Its unique design enhances BP1003 stability, cellular uptake, and target affinity. BP1003 efficiently reduces STAT3 expression and enhances the sensitivity of breast cancer cells (HER2+, triple negative) and ovarian cancer cells (late stage, invasive ovarian cancer) to paclitaxel and 5-fluorouracil (5-FU) in both 2D and 3D cell cultures. Similarly, ex vivo and in vivo patient-derived models of pancreatic ductal adenocarcinoma (PDAC) show reduced tissue viability and tumor volume with BP1003 and gemcitabine combination treatments. In addition to directly affecting tumor cells, BP1003 can modulate the tumor microenvironment. Unlike M1 differentiation, monocyte differentiation into anti-inflammatory M2 macrophages is suppressed by BP1003, indicating its potential contribution to immunotherapy. The broad anti-tumor effect of BP1003 in numerous preclinical solid tumor models, such as breast, ovarian, and pancreatic cancer models shown in this work, makes it a promising cancer therapeutic.
60
项与 BP-1003 相关的新闻(医药)2025-06-03
Highlights Promise of DNAbilize® Platform to Produce New Drug Candidates and Potential Licensing Opportunities HOUSTON, June 03, 2025 (GLOBE NEWSWIRE) -- Bio-Path Holdings, Inc., (NASDAQ:BPTH), a biotechnology company leveraging its proprietary DNAbilize® liposomal delivery and antisense technology to develop a portfolio of targeted nucleic acid cancer and obesity drugs, today announced highlights from the recent clinical development and operational update conference call and webcast held May 26, 2025. An archived webcast of the event can be accessed here. “Our business model centers around generating new drug candidates from our DNAbilize® platform and licensing them for final development and commercialization with partners that have expertise and scale to successfully bring them to market,” said Peter Nielsen, President and Chief Executive Officer of Bio-Path Holdings. “The clinical progress we are making across our pipeline is bringing us one step closer to our goal of delivering a better path for oncologic and cardiometabolic patients.” Clinical Program Overview Prexigebersen Phase 2 Clinical Trial – Bio-Path’s Phase 2 clinical trial for the treatment of AML is comprised of three cohorts of patients and treatments, each separately approvable by the FDA as a new indication. The first two cohorts are treating patients with the triple combination of prexigebersen, decitabine and venetoclax. The first cohort includes untreated AML patients, and the second cohort includes relapsed/refractory AML patients. Finally, the third cohort is treating relapsed/refractory AML patients who are venetoclax-resistant or intolerant with the two-drug combination of prexigebersen and decitabine. Outcomes for these older patients who are unable to receive intensive chemotherapy due to the challenging side effect profile, remain suboptimal with a median survival of only 5 to 10 months. As previously reported, Bio-Path identified two patients who have demonstrated continued treatment durability. These patients have each received over 15 treatment cycles and remain in complete remission. Bio-Path expects to utilize an advisory panel of AML experts to assist in the design of the final clinical development plans through potential FDA approval. Other significant milestones expected during 2025 include the completion of Cohort 2 and an interim analysis for Cohort 3. Phase 1/1b Clinical Trial in BP1001-A in Advanced Solid Tumors – A Phase 1/1b clinical trial of BP1001-A in patients with advanced or recurrent solid tumors, including ovarian and uterine, pancreatic and breast cancer, is ongoing. BP1001-A is a modified product candidate that incorporates the same drug substance as prexigebersen but has a slightly modified formulation designed to enhance nanoparticle properties. The Phase 1 study has advanced to the second, higher dose level and the first patient in the second dose cohort continued experiencing a positive response which may signal that this analog of prexigebersen has potential as a new treatment for advanced solid tumors. The patient continues to be doing well after failing extensive chemotherapy and surgical treatment for gynecologic cancer, demonstrating a 15% reduction in her primary tumor through ten cycles of treatment. Moreover, it appears that these positive outcomes may have contributed to allowing her to continue with rigorous exercise and improved quality of life. Completion of the second and third dosing cohorts are expected later this year. The Phase 1b portion of the study is expected to commence after successful completion of the three BP1001-A monotherapy dose level cohorts and is intended to assess the safety and efficacy of BP1001-A in combination with paclitaxel in patients with recurrent ovarian or endometrial tumors. Phase 1b studies are also expected to be initiated in combination with gemcitabine in Stage 4 pancreatic cancer and combination therapy in breast cancer. Phase 1/1b Clinical Trial in BP1002 in Relapsed/Refractory AML – A Phase 1/1b clinical trial for BP1002 to treat relapsed/refractory AML patients, including venetoclax-resistant patients, is ongoing. BP1002 targets the protein Bcl-2, which is responsible for driving cell survival in up to 60% of all cancers. Venetoclax treats AML patients by blocking the activity of the Bcl-2 protein in AML patients. However, over time patients become resistant to venetoclax. BP1002 treats the Bcl-2 target by blocking the cell’s ability to produce Bcl-2 and could provide benefit for these venetoclax resistant patients. AML patients that fail frontline venetoclax-based therapy have very poor prognosis with median overall survival of less than three months. The first dose cohort consisted of a starting dose of 20 mg/m2, the second dose cohort of 40 mg/m2 and there were no dose limiting toxicities. The third dosing cohort of 60 mg/m2 has been completed and the fourth dosing cohort of 90 mg/m2 is open for enrollment. Enrollment in the third dosing cohort closed faster than expected which Bio-Path believes reflects the need for additional treatment options. Prexigebersen as Potential Treatment for Obesity in Type 2 Diabetes Patients – BP1001-A downregulates Grb2 expression to increase insulin sensitivity and helps lower blood glucose level in Type 2 diabetes patients. Scientific evidence suggests that by downregulating Grb2 expression, BP1001-A could help lower blood glucose levels by affecting insulin signaling. Bio-Path conducted preclinical studies that confirmed the effectiveness of BP1001-A in affecting insulin signaling and its potential efficacy as a therapeutic treatment for obese patients who have Type 2 diabetes. In May, the Company reported the achievement of a third milestone from preclinical studies of BP1001-A that provide additional support for its potential as a treatment for obesity. These studies showed BP1001-A rescues the decrease in AKT activity in liver cells and prevents cells from becoming insulin resistant, confirming its potential as a treatment for obesity and related metabolic diseases in Type 2 diabetes patients. In 2025, Bio-Path expects to complete preclinical testing and to file an Investigational New Drug (IND) application. Intellectual Property Protection Bio-Path’s composition of matter patents are designed to protect encroachment from third parties on its proprietary products. These composition patents allow the Company to apply its core technology to new protein targets and receive new 20-year patents. Bio-Path’s patent portfolio is as follows: Composition and methods of use patents issued cover DNAbilize technology, solely owned by Bio-Path.Seven patents issued in the U.S. with one additional application allowed; 61 foreign patents issued across 26 countries; five additional foreign patent applications allowed; three applications pending in the U.S. along with more than 30 applications pending in foreign jurisdictions. About Bio-Path Holdings, Inc. Bio-Path is a biotechnology company developing DNAbilize®, a novel technology that has yielded a pipeline of RNAi nanoparticle drugs that can be administered with a simple intravenous infusion. Bio-Path’s lead product candidate, prexigebersen (BP1001, targeting the Grb2 protein), is in a Phase 2 study for blood cancers, and BP1001-A, a drug product modification of prexigebersen, is in a Phase 1/1b study for solid tumors. The Company’s second product, BP1002, which targets the Bcl-2 protein, is being evaluated for the treatment of blood cancers and solid tumors, including acute myeloid leukemia. In addition, an IND application is expected to be filed for BP1003, a novel liposome-incorporated STAT3 antisense oligodeoxynucleotide developed by Bio-Path as a specific inhibitor of STAT3. For more information, please visit the Company's website at http://www.biopathholdings.com. Forward-Looking Statements This press release contains forward-looking statements that are made pursuant to the safe harbor provisions of the federal securities laws. These statements are based on management's current expectations and accordingly are subject to uncertainty and changes in circumstances. Any express or implied statements contained in this press release that are not statements of historical fact may be deemed to be forward-looking statements. Any statements that are not historical facts contained in this release are forward-looking statements that involve risks and uncertainties, including Bio-Path’s ability to raise needed additional capital on a timely basis in order for it to continue its operations, have success in the clinical development of its technologies, the timing of enrollment and release of data in such clinical studies, the accuracy of such data, limited patient populations of early stage clinical studies and the possibility that results from later stage clinical trials with much larger patient populations may not be consistent with earlier stage clinical trials, the maintenance of intellectual property rights, that patents relating to existing or future patent applications will be issued or that any issued patents will provide meaningful protection of our drug candidates, the impact, risks and uncertainties related to global pandemics, including the COVID-19 pandemic, and actions taken by governmental authorities or others in connection therewith, and such other risks which are identified in Bio-Path's most recent Annual Report on Form 10-K, in any subsequent quarterly reports on Form 10-Q and in other reports that Bio-Path files with the Securities and Exchange Commission from time to time. These documents are available on request from Bio-Path Holdings or at www.sec.gov. Bio-Path disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. Contact Information: Investors Will O’Connor Stern Investor Relations, Inc.212-362-1200will@sternir.com Doug Morris Investor Relations Bio-Path Holdings, Inc. 832-742-1369
临床1期临床2期
2025-01-10
Advancing Multiple Programs in Areas of Significant Unmet Medical Need Several Milestones Across Clinical Development Pipeline Expected in 2025 HOUSTON, Jan. 10, 2025 (GLOBE NEWSWIRE) -- Bio-Path Holdings, Inc., (NASDAQ:BPTH), a biotechnology company leveraging its proprietary DNAbilize® liposomal delivery and antisense technology to develop a portfolio of targeted nucleic acid cancer and obesity drugs, today provides a clinical development and operational update for 2025. “The important work we conducted throughout 2024 has led us into what we believe is an exciting 2025 as we build off positive data generated in oncology and the addition of a new application for BP1001-A in the treatment of obesity for Type 2 Diabetes. This new application compels us to advance these studies as quickly as possible and to file for regulatory designations that could accelerate our path to approval,” said Peter H. Nielsen, President and Chief Executive Officer of Bio-Path. “There is no greater challenge than the battle against cancer, and developing effective new medicines for patients suffering with few treatment options is what drives us every day. The addition of development of a potential treatment for obesity in Type 2 Diabetes patients follows this pathway as these patients need treatment beyond current weight loss drugs to support needed therapy for reducing glucose levels, which has positive impact across a number of different health-related conditions. The substantial progress we have made gives us further confidence that our DNAbilize® platform is ushering in a new path of DNA-powered medicines that can make a difference in the lives of these patients.” Clinical Program Overview Bio-Path’s clinical development program consists of one Phase 2 clinical trial, two Phase 1 or 1/1b clinical trials, and two preclinical programs. Bio-Path has developed a molecular biomarker package for its Phase 2 clinical trial in acute myeloid leukemia (AML) that was developed to identify patients that potentially have a higher propensity to respond to prexigebersen treatment. Bio-Path expects to utilize the biomarker package to accompany prexigebersen treatment in 2025 and expects to evaluate prexigebersen for the treatment of obesity. In addition, BP1001-A is in preclinical development for treatment of obesity in Type 2 Diabetes patients, which may be submitted to the U.S. Food and Drug Administration (FDA) later in the year in an Investigational New Drug (IND) application. Development of Molecular Biomarkers – Bio-Path has developed a molecular biomarker package to accompany prexigebersen treatment, the goal of which is to identify patients with a genetic profile more likely to respond to treatment thereby improving probability of success for this program. The emerging role of biomarkers has been enhancing cancer development over the past decade and has become a more common companion to many cancer development programs. Bio-Path expects to utilize the prexigebersen biomarkers in 2025 in the Phase 2 AML clinical trial and to develop additional molecular biomarker packages to accompany its new programs. Prexigebersen Phase 2 Clinical Trial – Bio-Path’s Phase 2 clinical trial for the treatment of AML is comprised of three cohorts of patients and treatments, each separately approvable by the FDA as a new indication. The first two cohorts are treating patients with the triple combination of prexigebersen, decitabine and venetoclax. The first cohort includes untreated AML patients, and the second cohort includes relapsed/refractory AML patients. Finally, the third cohort is treating relapsed/refractory AML patients who are venetoclax-resistant or intolerant with the two-drug combination of prexigebersen and decitabine. Outcomes for these older patients who are unable to receive intensive chemotherapy due to the challenging side effect profile, remain suboptimal with a median survival of only 5 to 10 months. Bio-Path also expects to utilize an advisory panel of AML experts to assist in the design of the final clinical development plans through potential FDA approval. Other significant milestones expected during 2025 include the completion of Cohort 2 and an interim analysis for Cohort 3. Phase 1/1b Clinical Trial in BP1001-A in Advanced Solid Tumors – A Phase 1/1b clinical trial of BP1001-A in patients with advanced or recurrent solid tumors, including ovarian and uterine, pancreatic and breast cancer, is ongoing. BP1001-A is a modified product candidate that incorporates the same drug substance as prexigebersen but has a slightly modified formulation designed to enhance nanoparticle properties. The Phase 1 study has advanced to the second, higher dose level and the first patient in the second dose cohort continued experiencing a positive response which may signal that this analog of prexigebersen has potential as a new treatment for advanced solid tumors. The patient continues to be doing well after ten months on study after failing extensive chemotherapy and surgical treatment for gynecologic cancer, demonstrating a 15% reduction in her primary tumor through nine cycles of treatment. Moreover, it appears that these positive outcomes may have contributed to allowing her to continue with rigorous exercise and improved quality of life. Completion of the second and third dosing cohorts are expected in 2025. The Phase 1b portion of the study is expected to commence after successful completion of the three BP1001-A monotherapy dose level cohorts and is intended to assess the safety and efficacy of BP1001-A in combination with paclitaxel in patients with recurrent ovarian or endometrial tumors. Phase 1b studies are also expected to be initiated in combination with gemcitabine in Stage 4 pancreatic cancer and combination therapy in breast cancer. Phase 1/1b Clinical Trial in BP1002 in Relapsed/Refractory AML – A Phase 1/1b clinical trial for BP1002 to treat relapsed/refractory AML patients, including venetoclax-resistant patients, is ongoing. BP1002 targets the protein Bcl-2, which is responsible for driving cell survival in up to 60% of all cancers. Venetoclax treats AML patients by blocking the activity of the Bcl-2 protein in AML patients. However, over time patients become resistant to venetoclax. BP1002 treats the Bcl-2 target by blocking the cell’s ability to produce Bcl-2 and could provide benefit for these venetoclax resistant patients. AML patients that fail frontline venetoclax-based therapy have very poor prognosis with median overall survival of less than three months. The first dose cohort consisted of a starting dose of 20 mg/m2, the second dose cohort of 40 mg/m2 and there were no dose limiting toxicities. The third dosing cohort of 60 mg/m2 is open and enrollment closed faster than expected which Bio-Path believes reflects the need for additional treatment options. Prexigebersen as Potential Treatment for Obesity in Type 2 Diabetes Patients – BP1001-A downregulates Grb2 expression to increase insulin sensitivity and helps lower blood glucose level in Type 2 diabetes patients. Scientific evidence suggests that by downregulating Grb2 expression, BP1001-A could help lower blood glucose levels by affecting insulin signaling. Bio-Path conducted preclinical studies that confirmed the effectiveness of BP1001-A in affecting insulin signaling and its potential efficacy as a therapeutic treatment for obese patients who have Type 2 diabetes. In 2025, Bio-Path expects to complete preclinical testing and to file an IND. Intellectual Property Protection Bio-Path’s composition of matter patents are designed to protect encroachment from third parties on its proprietary products. These composition patents allow the Company to apply its core technology to new protein targets and receive new 20-year patents. Bio-Path’s patent portfolio is as follows: Composition and methods of use patents issued cover DNAbilize technology, solely owned by Bio-Path.Seven patents issued in the U.S. with one additional application allowed; 61 foreign patents issued across 24 countries; five additional foreign patent applications allowed; three applications pending in the U.S. along with more than 30 applications pending in foreign jurisdictions. About Bio-Path Holdings, Inc. Bio-Path is a biotechnology company developing DNAbilize®, a novel technology that has yielded a pipeline of RNAi nanoparticle drugs that can be administered with a simple intravenous infusion. Bio-Path’s lead product candidate, prexigebersen (BP1001, targeting the Grb2 protein), is in a Phase 2 study for blood cancers, and BP1001-A, a drug product modification of prexigebersen, is in a Phase 1/1b study for solid tumors. The Company’s second product, BP1002, which targets the Bcl-2 protein, is being evaluated for the treatment of blood cancers and solid tumors, including acute myeloid leukemia. In addition, an IND application is expected to be filed for BP1003, a novel liposome-incorporated STAT3 antisense oligodeoxynucleotide developed by Bio-Path as a specific inhibitor of STAT3. For more information, please visit the Company's website at http://www.biopathholdings.com. Forward-Looking Statements This press release contains forward-looking statements that are made pursuant to the safe harbor provisions of the federal securities laws. These statements are based on management's current expectations and accordingly are subject to uncertainty and changes in circumstances. Any express or implied statements contained in this press release that are not statements of historical fact may be deemed to be forward-looking statements. Any statements that are not historical facts contained in this release are forward-looking statements that involve risks and uncertainties, including Bio-Path’s ability to raise needed additional capital on a timely basis in order for it to continue its operations, have success in the clinical development of its technologies, the timing of enrollment and release of data in such clinical studies, the accuracy of such data, limited patient populations of early stage clinical studies and the possibility that results from later stage clinical trials with much larger patient populations may not be consistent with earlier stage clinical trials, the maintenance of intellectual property rights, that patents relating to existing or future patent applications will be issued or that any issued patents will provide meaningful protection of our drug candidates, the impact, risks and uncertainties related to global pandemics, including the COVID-19 pandemic, and actions taken by governmental authorities or others in connection therewith, and such other risks which are identified in Bio-Path's most recent Annual Report on Form 10-K, in any subsequent quarterly reports on Form 10-Q and in other reports that Bio-Path files with the Securities and Exchange Commission from time to time. These documents are available on request from Bio-Path Holdings or at www.sec.gov. Bio-Path disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. Contact Information:
Investors Will O’Connor Stern Investor Relations, Inc.212-362-1200will@sternir.com
Doug Morris Investor Relations Bio-Path Holdings, Inc. 832-742-1369
临床2期临床1期
2024-12-19
Preclinical Studies Confirmed BP1001-A Mechanism of Action and Therapeutic Potential in Obesity and Type 2 DiabetesHOUSTON, Dec. 19, 2024 (GLOBE NEWSWIRE) -- Bio-Path Holdings, Inc., (NASDAQ:BPTH), a biotechnology company leveraging its proprietary DNAbilize® liposomal delivery and antisense technology to develop a portfolio of targeted nucleic acid cancer drugs, today reported that results from preclinical studies of BP1001-A for obesity demonstrated enhanced insulin sensitivity, confirming BP1001-A as a potential treatment for obesity and related metabolic diseases in Type 2 diabetes patients. BP1001-A downregulates growth factor receptor-bound protein 2 (Grb2) expression to increase insulin sensitivity and helps lower blood glucose level in Type 2 diabetes patients. Scientific evidence suggests that by downregulating Grb2 expression, BP1001-A could help lower blood glucose level by affecting insulin signaling. Bio-Path conducted preclinical studies that confirmed the effectiveness of BP1001-A in affecting insulin signaling and its potential efficacy as a therapeutic treatment for obese patients who have Type 2 diabetes. The study results showed: BP1001-A reduced Grb2 protein expression in myoblast cellsBP1001-A increased the levels of phosphorylated AKT and phosphorylated FOXO-1 in myoblast and hepatoma cells in the presence of insulin These initial data confirmed that by downregulating Grb2 expression, BP1001-A could enhance insulin-induced metabolic events by affecting the insulin/phosphoinositol-3 kinase (PI3K)/AKT pathway and increase insulin sensitivity. “The success of our initial preclinical testing, which supports the mechanism of action and highlights the efficacy of BP1001-A to enhance insulin sensitivity, further validates BP1001-A as a potential treatment for obesity in Type 2 diabetes patients. The failure of leading weight loss medications to induce weight loss in obese patients who have Type 2 diabetes creates a compelling need for an alternative method of lowering blood glucose in obese patients who have Type 2 diabetes,” said Peter H. Nielsen, President and Chief Executive Officer of Bio-Path. “We are excited by the rapid progress we have made advancing BP1001-A as a potential treatment for obesity and related metabolic diseases in Type 2 diabetes patients based on previous BP1001-A preclinical studies as they support our continued and rapid development of this promising program.” Bio-Path has initiated animal studies to confirm the efficacy of BP1001-A as a potential treatment for obesity and related metabolic diseases in Type 2 diabetes patients. If successful, Bio-Path anticipates initiating a first-in-human Phase 1 clinical trial in 2025 to further validate safety, measure pharmacokinetics and establish dosing for potential pivotal trials. About Bio-Path Holdings, Inc. Bio-Path is a biotechnology company developing DNAbilize®, a novel technology that has yielded a pipeline of RNAi nanoparticle drugs that can be administered with a simple intravenous infusion. Bio-Path’s lead product candidate, prexigebersen (BP1001, targeting the Grb2 protein), is in a Phase 2 study for blood cancers, and BP1001-A, a drug product modification of prexigebersen, is in a Phase 1/1b study for solid tumors. The Company’s second product, BP1002, which targets the Bcl-2 protein, is being evaluated for the treatment of blood cancers and solid tumors, including acute myeloid leukemia. In addition, an IND application is expected to be filed for BP1003, a novel liposome-incorporated STAT3 antisense oligodeoxynucleotide developed by Bio-Path as a specific inhibitor of STAT3. For more information, please visit the Company's website at http://www.biopathholdings.com. Forward-Looking Statements This press release contains forward-looking statements that are made pursuant to the safe harbor provisions of the federal securities laws. These statements are based on management's current expectations and accordingly are subject to uncertainty and changes in circumstances. Any express or implied statements contained in this press release that are not statements of historical fact may be deemed to be forward-looking statements. Any statements that are not historical facts contained in this release are forward-looking statements that involve risks and uncertainties, including Bio-Path’s ability to raise needed additional capital on a timely basis in order for it to continue its operations, have success in the clinical development of its technologies, the timing of enrollment and release of data in such clinical studies, the accuracy of such data, limited patient populations of early stage clinical studies and the possibility that results from later stage clinical trials with much larger patient populations may not be consistent with earlier stage clinical trials, the maintenance of intellectual property rights, that patents relating to existing or future patent applications will be issued or that any issued patents will provide meaningful protection of our drug candidates, the impact, risks and uncertainties related to global pandemics, including the COVID-19 pandemic, and actions taken by governmental authorities or others in connection therewith, and such other risks which are identified in Bio-Path's most recent Annual Report on Form 10-K, in any subsequent quarterly reports on Form 10-Q and in other reports that Bio-Path files with the Securities and Exchange Commission from time to time. These documents are available on request from Bio-Path Holdings or at www.sec.gov. Bio-Path disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. Contact Information:
Investors Will O’Connor Stern Investor Relations, Inc.212-362-1200will@sternir.com
Doug Morris Investor Relations Bio-Path Holdings, Inc. 832-742-1369
临床2期临床结果
100 项与 BP-1003 相关的药物交易
登录后查看更多信息
研发状态
10 条进展最快的记录, 后查看更多信息
登录
| 适应症 | 最高研发状态 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|---|
| 肺癌 | 临床申请 | 美国 | 2024-12-01 | |
| 胰腺癌 | 临床申请 | 美国 | 2024-12-01 | |
| 乳腺癌 | 临床前 | 美国 | 2022-04-12 | |
| 卵巢囊肿 | 临床前 | 美国 | 2022-04-12 |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
No Data | |||||||
登录后查看更多信息
转化医学
使用我们的转化医学数据加速您的研究。
登录
或

药物交易
使用我们的药物交易数据加速您的研究。
登录
或

核心专利
使用我们的核心专利数据促进您的研究。
登录
或

临床分析
紧跟全球注册中心的最新临床试验。
登录
或

批准
利用最新的监管批准信息加速您的研究。
登录
或

特殊审评
只需点击几下即可了解关键药物信息。
登录
或

生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用

