预约演示
更新于:2025-07-31
Boltbody ISAC PD-L1(Bolt Bio)
更新于:2025-07-31
概要
基本信息
药物类型 免疫刺激抗体偶联药物(ISAC) |
别名 PD-L1 ISACs(Bolt Biotherapeutics) |
作用方式 抑制剂、激动剂 |
作用机制 PDL1抑制剂(程序性死亡配体1抑制剂)、TLR7激动剂(Toll样受体7激动剂)、TLR8激动剂(Toll样受体8激动剂) |
治疗领域 |
在研适应症 |
非在研适应症- |
非在研机构- |
权益机构- |
最高研发阶段临床前 |
首次获批日期- |
最高研发阶段(中国)- |
特殊审评- |
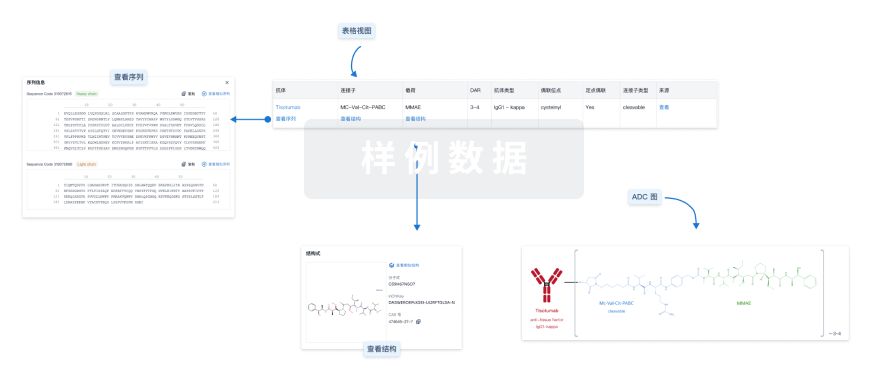
结构/序列
使用我们的ADC技术数据为新药研发加速。
登录
或
关联
100 项与 Boltbody ISAC PD-L1(Bolt Bio) 相关的临床结果
登录后查看更多信息
100 项与 Boltbody ISAC PD-L1(Bolt Bio) 相关的转化医学
登录后查看更多信息
100 项与 Boltbody ISAC PD-L1(Bolt Bio) 相关的专利(医药)
登录后查看更多信息
1
项与 Boltbody ISAC PD-L1(Bolt Bio) 相关的新闻(医药)2025-05-12
BDC-4182 Phase 1 dose-escalation study now open for enrollment BDC-3042 Phase 1 dose-escalation data presented at AACR 2025 demonstrated a favorable safety profile, dose-dependent biological activity, and monotherapy anti-tumor activity Bolt is running a partnering process to advance development of BDC-3042 Cash balance of $58.0 million as of March 31, 2025 anticipated to fund key milestones through mid-2026
REDWOOD CITY, Calif., May 12, 2025 (GLOBE NEWSWIRE) -- Bolt Biotherapeutics (Nasdaq: BOLT), a clinical-stage biopharmaceutical company developing novel immunotherapies for the treatment of cancer, today reported financial results for the first quarter ended March 31, 2025, and provided a business update. “We continue to make progress advancing our pipeline of first-in-class, novel immunotherapies,” said Willie Quinn, Chief Executive Officer. “We recently presented promising early clinical data for BDC-3042 at AACR and launched a process to find a partner to accelerate BDC-3042 development. We also just opened enrollment for the first next-generation Boltbody™ ISAC in our pipeline, BDC-4182. We are excited to see what our ISAC platform technology can do for gastric and gastroesophageal cancer patients. We continue to be good stewards of capital as we execute against our mission to deliver new treatment options to patients with cancer.”
Recent Highlights and Anticipated Milestones
Clinical data from Phase 1 dose-escalation study of BDC-3042 presented at AACR Annual Meeting 2025 in April 2025. BDC-3042 is a proprietary agonist antibody that targets dectin-2, an immune-activating receptor expressed by tumor-associated macrophages (TAMs). This single-agent, dose-escalation Phase 1 clinical study is evaluating BDC-3042 in patients with metastatic or unresectable solid tumors including non-small cell lung cancer (NSCLC). The dose-escalation data support the selection of 10 mg/kg q2w as a recommended Phase 2 dose (RP2D), alongside potential exploration of other doses and schedules. The results support further clinical development in NSCLC and other post-immunotherapy settings, as patients previously treated with PD-(L)1 inhibitors appear to have more dectin-2 expression and may experience improved outcomes. Bolt is running a process to find a partner with resources to accelerate development and optimize commercialization. BDC-4182 Phase 1 study for patients with gastric and gastroesophageal cancer opened for enrollment in April 2025. BDC-4182 is a next-generation Boltbody™ ISAC clinical candidate targeting claudin 18.2, a clinically validated target in oncology with expression in gastric/gastroesophageal junction cancer, pancreatic cancer, and other tumor types. Preclinically, monotherapy treatment with BDC-4182 generated complete regressions in multiple models and BDC-4182 was tolerated in toxicology studies. BDC-4182 outperformed cytotoxic claudin 18.2 ADCs, using MMAE or TOPO1, in multiple preclinical studies. Presented preclinical results for next-generation Boltbody™ ISACs targeting CEA and PD-L1 at AACR Annual Meeting 2025 in April 2025. Bolt’s lead CEA-targeting ISAC comprises a novel, fully human antibody with high affinity and selectivity to CEACAM5 (CEA), and not to other members of the CEACAM family, conjugated to a propriety TLR7/8 agonist via a non-cleavable linker. This ISAC drives enhanced phagocytosis of CEA-positive tumor cells and stimulates production of critical immune-activating cytokines including IL-12p70, IFNg, and TNFa. The next-generation CEA ISAC induced complete and durable anti-tumor responses in mice and was well-tolerated in NHPs. Bolt’s PD-L1 ISAC utilizes a novel human anti-PD-L1 antibody conjugated to a TLR7/8 agonist via a non-cleavable linker. This ISAC leverages a unique mechanism of action due to its ability to target both tumor and immune cells that express PD-L1. Preclinical results demonstrated that PD-L1 ISACs represent a compelling new approach to treat cancer, leveraging mechanisms that are distinct from and potentially complementary to conventional PD-1/PD-L1 blockade. Collaborations with Genmab and Toray continue to progress. Genmab and Bolt have now selected a second product to advance into development, while the companies also continue research and development on additional programs. The Toray collaboration combines the Company’s immunostimulatory linker-payloads with Toray antibodies targeting Caprin-1, a tumor-specific antigen that is strongly expressed on the cell membrane in multiple solid tumor types. Cash, cash equivalents, and marketable securities were $58.0 million as of March 31, 2025. Cash on hand is expected to fund multiple milestones and operations through mid-2026.
Bolt’s lead CEA-targeting ISAC comprises a novel, fully human antibody with high affinity and selectivity to CEACAM5 (CEA), and not to other members of the CEACAM family, conjugated to a propriety TLR7/8 agonist via a non-cleavable linker. This ISAC drives enhanced phagocytosis of CEA-positive tumor cells and stimulates production of critical immune-activating cytokines including IL-12p70, IFNg, and TNFa. The next-generation CEA ISAC induced complete and durable anti-tumor responses in mice and was well-tolerated in NHPs. Bolt’s PD-L1 ISAC utilizes a novel human anti-PD-L1 antibody conjugated to a TLR7/8 agonist via a non-cleavable linker. This ISAC leverages a unique mechanism of action due to its ability to target both tumor and immune cells that express PD-L1. Preclinical results demonstrated that PD-L1 ISACs represent a compelling new approach to treat cancer, leveraging mechanisms that are distinct from and potentially complementary to conventional PD-1/PD-L1 blockade.
First Quarter 2025 Financial Results
Collaboration Revenue – Total collaboration revenue was $1.2 million for the quarter ended March 31, 2025, compared to $5.3 million for the same quarter in 2024. Revenue in the comparative periods was generated from services performed under the R&D collaborations as we fulfill our performance obligations. The decrease in revenue in the comparative period was mainly due to the $3.5 million revenue recognized under the Amended Innovent Agreement, as we completed our performance obligation to Innovent. Research and Development (R&D) Expenses – R&D expenses were $9.5 million for the quarter ended March 31, 2025, compared to $16.5 million for the same quarter in 2024. The decrease between the comparable periods was mainly due to a decrease in salary and related expenses, a decrease in clinical expenses primarily related to the discontinued development of trastuzumab imbotolimod, formerly known as BDC-1001 in May 2024. General and Administrative (G&A) Expenses – G&A expenses were $3.8 million for the quarter ended March 31, 2025, compared to $5.8 million for the same quarter in 2024. The decrease between the comparable periods was mainly due to a decrease in salary and related expenses primarily as a result of the May 2024 restructuring. Loss from Operations – Loss from operations was $12.1 million for the quarter ended March 31, 2025, compared to $17.1 million for the same quarter in 2024.
About the Boltbody™ Immune-Stimulating Antibody Conjugate (ISAC) PlatformBolt Biotherapeutics’ Boltbody ISAC platform harnesses the precision of antibodies with the power of the innate and adaptive immune system to generate a productive anti-cancer response. Each Boltbody ISAC candidate comprises a tumor-targeting antibody, a non-cleavable linker, and a proprietary immune stimulant. The antibody is designed to target one or more markers on the surface of a tumor cell and the immune stimulant is designed to recruit and activate myeloid cells. Activated myeloid cells initiate a positive feedback loop by releasing cytokines and chemokines, chemical signals that attract other immune cells and lower the activation threshold for an immune response. This increases the population of activated immune system cells in the tumor microenvironment and promotes a robust immune response with the goal of generating durable therapeutic responses for patients with cancer.
About Bolt Biotherapeutics, Inc. Bolt Biotherapeutics is a clinical-stage biopharmaceutical company developing novel immunotherapies for the treatment of cancer. Bolt Biotherapeutics’ pipeline candidates are built on the Company’s deep expertise in myeloid biology and cancer drug development. The Company’s pipeline includes BDC-3042, a first-in-class agonist antibody that activates macrophages by targeting dectin-2, and BDC-4182, a next-generation Boltbody™ Immune-Stimulating Antibody Conjugate (ISAC) clinical candidate targeting claudin 18.2. BDC-3042 is currently in a Phase 1 dose escalation trial that includes patients with any of seven different solid tumor types. BDC-4182 is supported by strong in vitro and in vivo data demonstrating potent anti-tumor activity, and activities are underway to support the initiation of clinical trials in the second quarter of 2025. Bolt Biotherapeutics is also developing additional Boltbody™ ISACs in strategic collaborations with leading biopharmaceutical companies. For more information, please visit https://www.boltbio.com/.
Forward-Looking StatementsThis press release contains forward-looking statements about us and our industry that involve substantial risks and uncertainties and are based on our beliefs and assumptions and on information currently available to us. All statements other than statements of historical facts contained in this press release, including statements regarding the advancement and success of our BDC-3042 clinical trial, the potential initiation of clinical trials for BDC-4182, the anti-tumor potency, safety and tolerability, and characteristics of our product candidates, the initiation of future clinical trials, the potential value of collaborations, and the expected duration of our cash runway, are forward-looking statements. In some cases, you can identify forward-looking statements because they contain words such as “anticipate,” “believe,” “could,” “estimate,” “expect,” “intend,” “may,” “on track,” “plan,” “potential,” “predict,” “project,” “should,” “will,” or “would,” or the negative of these words or other similar terms or expressions. Forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance, or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Forward-looking statements represent our current beliefs, estimates and assumptions only as of the date of this press release and information contained in this press release should not be relied upon as representing our estimates as of any subsequent date. These statements, and related risks, uncertainties, factors and assumptions, include, but are not limited to: the potential product candidates that we develop may not progress through clinical development or receive required regulatory approvals within expected timelines or at all; clinical trials may not confirm any safety, potency or other product characteristics described or assumed in this press release; such product candidates may not be beneficial to patients or become commercialized; and our ability to maintain our current collaborations and establish further collaborations. These risks are not exhaustive. Except as required by law, we assume no obligation to update these forward-looking statements, or to update the reasons actual results could differ materially from those anticipated in the forward-looking statements, even if new information becomes available in the future. Further information on factors that could cause actual results to differ materially from the results anticipated by our forward-looking statements is included in the reports we have filed or will file with the Securities and Exchange Commission, including our Annual Report on Form 10-K for the year ended December 31, 2024. These filings, when available, are available on the investor relations section of our website at investors.boltbio.com and on the SEC’s website at www.sec.gov.
Investor Relations and Media Contact: Matthew DeYoung Argot Partners (212) 600-1902 boltbio@argotpartners.com
临床1期免疫疗法AACR会议财报
100 项与 Boltbody ISAC PD-L1(Bolt Bio) 相关的药物交易
登录后查看更多信息
研发状态
10 条进展最快的记录, 后查看更多信息
登录
| 适应症 | 最高研发状态 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|---|
| 肿瘤 | 临床前 | 美国 | 2025-04-30 |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
No Data | |||||||
登录后查看更多信息
转化医学
使用我们的转化医学数据加速您的研究。
登录
或

药物交易
使用我们的药物交易数据加速您的研究。
登录
或

核心专利
使用我们的核心专利数据促进您的研究。
登录
或

临床分析
紧跟全球注册中心的最新临床试验。
登录
或

批准
利用最新的监管批准信息加速您的研究。
登录
或

生物类似药
生物类似药在不同国家/地区的竞争态势。请注意临床1/2期并入临床2期,临床2/3期并入临床3期
登录
或

特殊审评
只需点击几下即可了解关键药物信息。
登录
或

生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用
