预约演示
Cellecta, Inc. Launches DriverMap™ Adaptive Immune Receptor (AIR) Human RNA Spike-In Controls to Ensure Consistent Quality and Optimal Performance of TCR and BCR Profiling Assays
2024-04-04
siRNA信使RNA寡核苷酸
The first-to-market DriverMap™ AIR RNA TCR/BCR spike-in calibration controls measure the accuracy, sensitivity, and linear range of sequencing-based immune repertoire profiling assays.
MOUNTAIN VIEW, Calif., April 4, 2024 /PRNewswire/ -- Cellecta, Inc. today announced the launch of the DriverMap™ Adaptive Immune Receptor (AIR) Human RNA Spike-In Premixed Controls, the first commercially available collection of synthetic mRNA constructs that can serve as universal controls for commercial or home-brewed AIR repertoire sequencing (AIR-Seq, TCR-seq and BCR-seq) assays based on multiplex RT-PCR or 5' RACE PCR techniques.
Continue Reading
Preview
来源: PRNewswire
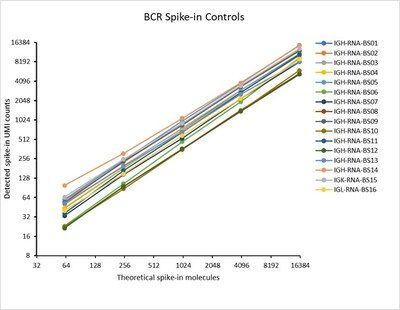
BCR Spike-in Control Mix was added to PMBC Control RNA (50 ng) at different input amounts (0.5µl, 2µl and 8 µl). DriverMap AIR Assay was run and the clonotypes were sequenced at 20M read per sample. MiXCR pipeline was used to calculate detected UMI as compared to spike-in amount (theoretical amount). Results show a linear trend for each construct as the input amount increases.

Preview
来源: PRNewswire
While AIR-Seq assays have been increasingly adopted in recent years to gain insight into the adaptive immune response, the lack of readily available controls has limited their utility and made harmonization of data across platforms next to impossible. Now, the possibility of adding validated RNA spike-in controls to an AIR assay provides a way to measure and check biases introduced by PCR, next-generation sequencing (NGS), cross-contamination and batch-to-batch experimental variation.
"We are pleased to offer the research community a new tool to optimize performance of different AIR-Seq assays and improve the quality of immune receptor repertoire profiling data," affirmed Alex Chenchik, Ph.D., president and chief scientific officer of Cellecta. "This should increase the utility of these assays for discovery of novel biomarkers and ensure reliable results that will afford greater insights into immunity mechanisms for a wide range of human diseases."
The DriverMap™ AIR RNA Spike-In Controls' key characteristics include:
These controls consist of 48 B-Cell Receptor (BCR) and 39 T-Cell Receptor (TCR) synthetic mRNA constructs designed to mimic all the different and most abundant TCR and BCR genes.
When introduced into the sample, the synthetic controls are amplified and sequenced along with the endogenous TCRs and BCRs
, thus presenting a defined copy number of RNA transcripts for calibration and QC of NGS data.
The ability to standardize results across samples with these RNA spike-in constructs
ensures the accuracy of sequencing data and eliminates bias caused by PCR and sequencing.
These standards and controls will help attain the goal of more reliable and reproducible AIR sequencing (AIR-Seq) data harmonization, interpretation, and sharing.
In addition to offering the DriverMap AIR RNA Spike-In Controls, which are useful on all AIR-Seq assay platforms, Cellecta offers its own workflow, including AIR-DNA and AIR-RNA kits, a full TCR/BCR profiling service and a data analysis pipeline.
For more information on the DriveMap AIR RNA Spike-In Controls, including introductory pricing, visit www.cellecta.com/DriverMapAIR or email [email protected].
About Cellecta:
Cellecta, Inc. is a trusted provider of genomic products and services that advance researchers' drug target and biomarker discovery efforts. Since 2006, we have collaborated with the world's leading pharma, biotech, government, and academic institutions. Numerous scientific papers have been published utilizing Cellecta's expertise in viral vector production, phenotypic screening, custom cell engineering and multiplex RT-qPCR.
Cellecta, Inc. is headquartered in Mountain View, California. More information about the company and its products and services may be found at www.cellecta.com
Cellecta, Inc.
Paul Diehl, 650-938-4050
[email protected]
or
Media:
Ruth Mercado, 650-938-4080
[email protected]
SOURCE Cellecta, Inc.
更多内容,请访问原始网站
文中所述内容并不反映新药情报库及其所属公司任何意见及观点,如有版权侵扰或错误之处,请及时联系我们,我们会在24小时内配合处理。
机构
-适应症
-药物
-生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。


