预约演示
更新于:2025-12-06

University of L'Aquila
更新于:2025-12-06
概览
标签
肿瘤
泌尿生殖系统疾病
神经系统疾病
小分子化药
疾病领域得分
一眼洞穿机构专注的疾病领域
暂无数据
技术平台
公司药物应用最多的技术
暂无数据
靶点
公司最常开发的靶点
暂无数据
| 排名前五的药物类型 | 数量 |
|---|---|
| 小分子化药 | 4 |
关联
4
项与 University of L'Aquila 相关的药物靶点 |
作用机制 EphA2拮抗剂 |
在研适应症 |
非在研适应症- |
最高研发阶段临床前 |
首次获批国家/地区- |
首次获批日期- |
靶点 |
作用机制 XPO1抑制剂 |
非在研适应症- |
最高研发阶段临床前 |
首次获批国家/地区- |
首次获批日期- |
靶点 |
作用机制 XPO1抑制剂 |
最高研发阶段临床前 |
首次获批国家/地区- |
首次获批日期- |
63
项与 University of L'Aquila 相关的临床试验NCT07126353
Metabolic Risk Assessment in Prepubertal Children With Congenital Hypothyroidism (IpoMet)
We propose a multicenter prospective study to define the prevalence and severity score of metabolic syndrome in a prepubertal pediatric cohort with congenital hypothyroidism, compared to a healthy and normal-weight pediatric population. These data will help to define whether hypothyroidism can be considered a risk factor for the metabolic health of the pediatric population. The possible identification of an at-risk metabolic profile will provide useful information to optimize the diagnostic and monitoring pathway for affected children.
开始日期2025-09-01 |
申办/合作机构  Perugia Hospital Perugia Hospital [+6] |
NCT06525987
Telecoached Exercise Intervention for Connectivity Enhancement in Small Vessel Disease (TELECONNECT-SVD): a Randomized Clinical Trial
The TELECONNECT-SVD study is a prospective, randomized, multicenter trial aimed at testing the efficacy of a remotely delivered exercise protocol on brain functional connectivity in patients with small vessel disease (SVD)-related ischemic stroke. The study will recruit patients aged ≥60 with a history of lacunar stroke, minimal disability (modified Rankin Scale score 0-1), and low physical activity levels.
The trial will include 60 participants randomized 1:1 to either a 24-week telecoached exercise intervention or usual care. The exercise program consists of multicomponent physical exercises delivered remotely twice a week. Assessments will be conducted at baseline, 12 weeks, 24 weeks, and 48 weeks.
Primary outcomes include changes in brain functional connectivity assessed by high-density EEG and improvements in physical fitness measured by the Senior Fitness Test. Secondary outcomes encompass changes in physical activity levels, anthropometric measurements, and vital signs.
The study employs a "wait list" design, where the control group receives the intervention after the initial 24-week period. This approach allows for assessment of the intervention's immediate effects and the retention of benefits after cessation.
Key features of the protocol include:
* Use of telecoaching to enhance adherence to the exercise program
* Comprehensive assessment of brain connectivity using advanced EEG analysis techniques
* Focus on patients with SVD, who may benefit significantly from exercise interventions
* Evaluation of both neurophysiological and clinical outcomes
The study aims to provide evidence for the potential benefits of exercise in enhancing brain connectivity and physical fitness in SVD patients, potentially informing future treatment guidelines and preventive strategies for this population.
The trial will include 60 participants randomized 1:1 to either a 24-week telecoached exercise intervention or usual care. The exercise program consists of multicomponent physical exercises delivered remotely twice a week. Assessments will be conducted at baseline, 12 weeks, 24 weeks, and 48 weeks.
Primary outcomes include changes in brain functional connectivity assessed by high-density EEG and improvements in physical fitness measured by the Senior Fitness Test. Secondary outcomes encompass changes in physical activity levels, anthropometric measurements, and vital signs.
The study employs a "wait list" design, where the control group receives the intervention after the initial 24-week period. This approach allows for assessment of the intervention's immediate effects and the retention of benefits after cessation.
Key features of the protocol include:
* Use of telecoaching to enhance adherence to the exercise program
* Comprehensive assessment of brain connectivity using advanced EEG analysis techniques
* Focus on patients with SVD, who may benefit significantly from exercise interventions
* Evaluation of both neurophysiological and clinical outcomes
The study aims to provide evidence for the potential benefits of exercise in enhancing brain connectivity and physical fitness in SVD patients, potentially informing future treatment guidelines and preventive strategies for this population.
开始日期2025-05-02 |
申办/合作机构 |
NCT06823466
Advancing Knowledge in Ischemic Stroke Patients on Oral Anticoagulants - The ASPERA International Registry
The Advancing knowledge in ischemic Stroke PatiEnts on oRal Anticoagulants (ASPERA) study aims to investigate characteristics of ischemic stroke cases occurring in patients on oral anticoagulation for atrial fibrillation (AF) or other cardioembolic arrhythmias and to characterize short and long-term outcomes associated with different secondary prevention strategies to prevent stroke recurrences. The ASPERA study is a multicenter, observational, both retrospective and prospective real-world study involving acute ischemic stroke patients occurring on oral anticoagulation. The study will encompass a retrospective (ASPERA-R) and prospective (ASPERA-P) data collection. Patient will be recruited consecutively at different emergency services and stroke units worldwide. University of L'Aquila (UnivAQ) will be in charge of study coordination, data analysis and management. The duration of ASPERA-R will be of 5-year from the study initiation of the study. Participating centers will be given a 6-month timeframe to enter retrospective data, commencing from the date of study approval.
ASPERA-P duration will be of 2 years of enrollment from the study approval and follow-up of 5 years. (study conclusion after 7 years of approval). Inclusion criteria will be: 1.Confirmed diagnosis of ischemic stroke. 2. Availability of at least one neuroimaging exam positive for ischemic lesion(s) consistent with patient symptoms. 3. Ongoing oral anticoagulation at the time of the index ischemic stroke. 4. Prior diagnosis of atrial fibrillation or other cardioembolic arrhythmias. 5. Written informed consent provided by the patient himself or by proxy. Patients with Symptoms not indicative of acute stroke, ongoing intravenous or subcutaneous anticoagulation at the time of stroke will be excluded. ASPERA-R: characterization of demographic, clinical and neuroimaging features of ischemic stroke cases occurring on oral anticoagulants. The primary outcome will be: ASPERA-R : characterization of demographic, clinical and neuroimaging features of ischemic stroke cases occurring on oral anticoagulants. ASPERA-P: risk of ischemic stroke recurrence of ischemic stroke cases occurring on oral anticoagulants across different secondary preventive strategies (i.e., maintaining the same type of oral anticoagulation versus switching to a different secondary prevention strategy) at 90 days, 1 and 5 years after the index stroke. Additionally, the study will aim to investigate the risk of safety events (hemorrhagic transformation, intracranial hemorrhage, other major bleeding events, any bleeding events, death due to any cause), risk of other major ischemic events (transient ischemic attack, myocardial infarction, death due to vascular causes) at each follow-up and to identify demographic, clinical and neuroimaging features of ischemic stroke recurrences.
ASPERA-P duration will be of 2 years of enrollment from the study approval and follow-up of 5 years. (study conclusion after 7 years of approval). Inclusion criteria will be: 1.Confirmed diagnosis of ischemic stroke. 2. Availability of at least one neuroimaging exam positive for ischemic lesion(s) consistent with patient symptoms. 3. Ongoing oral anticoagulation at the time of the index ischemic stroke. 4. Prior diagnosis of atrial fibrillation or other cardioembolic arrhythmias. 5. Written informed consent provided by the patient himself or by proxy. Patients with Symptoms not indicative of acute stroke, ongoing intravenous or subcutaneous anticoagulation at the time of stroke will be excluded. ASPERA-R: characterization of demographic, clinical and neuroimaging features of ischemic stroke cases occurring on oral anticoagulants. The primary outcome will be: ASPERA-R : characterization of demographic, clinical and neuroimaging features of ischemic stroke cases occurring on oral anticoagulants. ASPERA-P: risk of ischemic stroke recurrence of ischemic stroke cases occurring on oral anticoagulants across different secondary preventive strategies (i.e., maintaining the same type of oral anticoagulation versus switching to a different secondary prevention strategy) at 90 days, 1 and 5 years after the index stroke. Additionally, the study will aim to investigate the risk of safety events (hemorrhagic transformation, intracranial hemorrhage, other major bleeding events, any bleeding events, death due to any cause), risk of other major ischemic events (transient ischemic attack, myocardial infarction, death due to vascular causes) at each follow-up and to identify demographic, clinical and neuroimaging features of ischemic stroke recurrences.
开始日期2025-02-12 |
申办/合作机构 |
100 项与 University of L'Aquila 相关的临床结果
登录后查看更多信息
0 项与 University of L'Aquila 相关的专利(医药)
登录后查看更多信息
10,749
项与 University of L'Aquila 相关的文献(医药)2026-05-01·Neural Regeneration Research
Neuroinflammation strokes the brain: A double-edged sword in ischemic stroke
Article
作者: Lombardozzi, Giorgia ; d’Angelo, Michele ; Giorgi, Chiara ; Castelli, Vanessa ; Cimini, Annamaria
Stroke is a major cause of death and disability worldwide. It is characterized by a highly interconnected and multiphasic neuropathological cascade of events, in which an intense and protracted inflammatory response plays a crucial role in worsening brain injury. Neuroinflammation, a key player in the pathophysiology of stroke, has a dual role. In the acute phase of stroke, neuroinflammation exacerbates brain injury, contributing to neuronal damage and blood–brain barrier disruption. This aspect of neuroinflammation is associated with poor neurological outcomes. Conversely, in the recovery phase following stroke, neuroinflammation facilitates brain repair processes, including neurogenesis, angiogenesis, and synaptic plasticity. The transition of neuroinflammation from a harmful to a reparative role is not well understood. Therefore, this review seeks to explore the mechanisms underlying this transition, with the goal of informing the development of therapeutic interventions that are both time- and context-specific. This review aims to elucidate the complex and dual role of neuroinflammation in stroke, highlighting the main actors, biomarkers of the disease, and potential therapeutic approaches.
2026-01-01·INTERNATIONAL JOURNAL OF CARDIOLOGY
Subcutaneous ICD sports safety registry (SISS registry)
Article
作者: Landra, Federico ; Scarà, Antonio ; Segreti, Luca ; Notarstefano, Pasquale ; Menichetti, Francesca ; Sciarra, Luigi ; Nesti, Martina ; Russo, Vincenzo ; Perini, Alessandro Paoletti ; Solarino, Gianluca ; Palamà, Zefferino ; Bartoli, Chiara ; Giaccardi, Marzia ; Rossi, Andrea
BACKGROUND:
Exercise has a positive impact on physical function and quality of life. However, individuals with an implantable cardioverter-defibrillator (ICD) may experience adverse events during sports activities. To date, scientific data on the safety of sports participation in patients with subcutaneous ICDs (S-ICDs) are lacking. This study aims to analyze the occurrence of appropriate and inappropriate arrhythmic events related to sports activity in a population of S-ICD patients.
METHODS:
Consecutive patients who underwent S-ICD implantation were enrolled. Baseline clinical data, as well as information on sports activity and arrhythmic events, were collected.
RESULTS:
Among 280 patients (median age 46 [32-55] years; 72 % male) included in the registry, 205 (73 %) engaged in sports activities. During a follow-up period of 38 ± 13 months, 27 patients (13 %) experienced appropriate shocks. Of these, 20 (74.1 %) occurred at rest and 7 (25.9 %) during peak performance in sports. All patients who received appropriate shocks were implanted for secondary prevention. Additionally, 27 patients (13.1 %) experienced inappropriate shocks, with 15 (55.6 %) occurring at rest and 12 (44.4 %) during peak physical activity, primarily due to T-wave oversensing. No significant differences were observed in type or intensity of sports activity between patients who experienced shocks and those who did not.
CONCLUSION:
In S-ICD patients, both appropriate and inappropriate shocks occur more frequently during peak physical performance but are not associated with the type or intensity of sports activity. Appropriate shocks were most common in patients with arrhythmogenic or ischemic cardiomyopathy, while inappropriate shocks were mainly due to T-wave oversensing.
2025-12-31·Systems Biology in Reproductive Medicine
Exploring ovarian aging: unraveling the link between mitochondria status and oocyte as a determinant of gamete quality
Review
作者: Khalili, Mohammad Ali ; Sansone, Luigi ; Russo, Matteo Antonio ; Gatti, Marta ; Nottola, Stefania Annarita ; Belli, Manuel ; Macchiarelli, Guido ; Palmerini, Maria Grazia
The variation in reproductive age among individuals is significant, with many cases of infertility involving premature ovarian aging. This issue, combined with the societal trend of delaying childbearing, leads to age-related ovarian dysfunction. Ovarian aging is related to a decline of ovarian reserve, as oocyte quantity, quality, and precocious senescence, and may affect fertility and the overall individual well-being. Mitochondria play a central role in the maintenance of any cell health. Then mitochondrial dysfunctions may be responsible also for a negative impact on the quality, number, and function of oocytes, leading to different age-related reproductive disorders, impaired oogenesis, and embryogenesis. Although a large number of researches have shown clearly that mitochondrial dysfunction and morphology changes affect the maintenance and function of all major organs and tissues, such as the brain, heart, skeletal muscle, liver, and others the mechanisms contributing to early ovarian aging, a decrease of oocyte quality, and infertility remain unclear. In this review, we summarize the role of mitochondrial dysfunction in ovarian aging, presenting recent findings on morpho-functional changes in these organelles, and highlighting how their dysfunction accelerates ovary and cell senescence. We also explore their impact on oocyte functions. The reported data highlight the critical role of mitochondria in maintaining and enhancing oocyte quality, indicating that future studies should further focus on the mechanisms underlying mitochondrial damage and on identifying mitochondrial targets that may offer promising strategies to preserve, recover, and extend fertility in aging women.
10
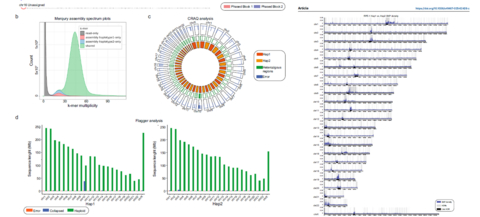
项与 University of L'Aquila 相关的新闻(医药)2025-10-10
Italian excellence with international partnerships
99.9999% accuracy across multiple chromosomes
Results published in Nature Communications
Boost to the study of centromeres
Use of Dante Omics genomic technology
RPE-1 is one of the three most widely used cell lines in the world
ROME--(BUSINESS WIRE)--A few weeks ago, the USCS Genome Browser at the University of California, Santa Cruz, the bible of reference genomics, published a new and very important reference genome, Diploid RPE-1, key to the study of centromeres, thanks to the work of the Area Science Park in Trieste, the Giunta Lab at Sapienza University, Dante Labs, and the University of L'Aquila. These innovative Italian institutions have formed an international alliance with Rockefeller University in New York and the University of Tennessee to successfully complete the complex genomic assembly.
Trieste, L’Aquila, Roma: il triangolo Italiano della genomica al servizio dell’umanità con Dante Labs, Area Science Park, UnivAQ e la Sapienza, grazie al nuovo genoma di riferimento diploide per la linea cellulare RPE-1
The RPE-1 line is not just any cell line, but one of the three most widely used in cell and molecular biology experiments worldwide, fueling discoveries in genetics, cancer research, and drug development. The team used innovative equipment such as PacBio and Oxford Nanopore technologies.
"Imagine trying to find an error in software when important files are missing from the source code," says Andrea Riposati, CEO of Dante Omics AI. "Now, we have an incredibly accurate source code for RPE-1 cells, which includes super important regions like centromeres. This is a breakthrough for all biotech companies involved in cell engineering and CRISPR gene editing."
The RPE1v1.1 assembly is available to the public via NCBI and UCSC Genome Browser.
The full article in Nature Communications: https://www.nature.com/articles/s41467-025-62428-z
More information at: danteomics.com
About Dante Omics AI
Dante Omics AI: a leader in AI-powered genomic and multi-omic solutions, dedicated to accelerating discovery and improving patient outcomes through advanced computational biology tools. The company's innovative platform combines cutting-edge artificial intelligence with high-performance computing to deliver transformative genomic analysis capabilities to research institutes, clinical laboratories, and biotechnology companies worldwide.
基因疗法寡核苷酸
2025-10-03
99.9999% accuracy on multiple chromosomes
Results published on Nature Communications
True diploid : both maternal + paternal DNA fully captured
Boost to the study of centromeres
Use of the Dante Labs Genome technology to advance diploid research
RPE-1 is one of the world’s top three most used cell lines
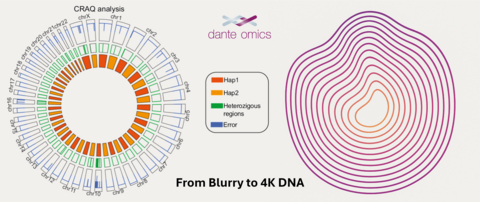
NEW YORK--(BUSINESS WIRE)--Dante Omics AI, Rockefeller University, Giunta Lab, Sapienza University, University of Tennessee, Trieste Area Science Park and University of L’Aquila published the successful assembly of a near-complete human diploid reference genome for one of the world’s most widely used cell lines.
From Blurry to 4K DNA: Dante Omics AI, Rockefeller University, Giunta Lab and global partners assemble the most complete genome for the RPE-1 cell line – the standard for complex cell engineering experiments.
This breakthrough provides a reference-quality blueprint of the RPE-1 cell line, a workhorse in laboratories around the globe. Dante Omics leveraged its expertise with the Dante Labs genome sequencing test and then partnered with top tier global researchers for this project.
“Imagine trying to debug software when the source code is missing critical lines,” says Andrea Riposati, CEO of Dante Omics AI. “Now we have a precise source code for RPE-1 cells—down to the most complex, previously hidden regions of DNA like centromeres. This new reference genome is a game changer for all biotech firms advancing cell engineering and CRISPR gene editing.”
The RPE-1 line is not just any cell line — it’s one of the top three most used in cellular and molecular biology experiments worldwide, powering discoveries in genetics, cancer research, and drug development. By building the first high-accuracy, fully phased diploid genome for RPE-1, the team has leveraged short reads and long reads sequencing including PacBio and Oxford Nanopore technologies.
“We thank the Giunta Lab and our partners for this exciting project,” says Andrea Riposati.
The RPE1v1.1 assembly is publicly available through NCBI and the UCSC Genome Browser, ensuring open access for researchers worldwide.
The full paper was published on Nature Communications: https://www.nature.com/articles/s41467-025-62428-z
More information at: danteomics.com
About Dante Omics AI
We are a leading provider of AI-powered genomics solutions, dedicated to accelerating discovery and improving patient outcomes through advanced computational biology tools. The company's innovative platform combines cutting-edge artificial intelligence with high-performance computing to deliver transformative genomic analysis capabilities for research institutions, clinical laboratories, and biotechnology companies worldwide.
寡核苷酸
2025-04-28
DOVER, Del., April 28, 2025 (GLOBE NEWSWIRE) -- Portage Biotech Inc. (NASDAQ: PRTG), a clinical-stage immuno-oncology company today reports confirmatory preclinical efficacy data for PORT-7 (TT-4), a selective adenosine A2B receptor inhibitor. Dr. Luciano Mutti of the Department of Applied Clinical Sciences and Biotechnology at the University of L'Aquila, Italy, an internationally recognized expert in mesothelioma, will be presenting the data at the American Association for Cancer Research® (AACR) Annual Meeting at the McCormick Place Convention Center in Chicago, Illinois on April 28, 2025. The new data in a murine mesothelioma model demonstrated single agent activity for PORT-7 that was superior to treatment with single agent anti-PD1 antibody. Moreover, the combination of PORT-7 and anti-PD1 was superior to treatment with either anti-PD1 or PORT-7 alone. Immunohistochemistry of the tumors revealed the formation of tertiary lymphoid structures in the mice receiving the combination. This indication of a favorable immune response was accompanied by increases in immune effector cells in mice treated with the combination. Mesothelioma is an aggressive cancer with limited treatment options in need of novel approaches to overcome immune resistance. Portage is making preparations to commence a first-in-human clinical trial with PORT-7. Combining PORT-6 and PORT-7 for a More Comprehensive Immunotherapy Approach In parallel, Portage is advancing the dose escalation of PORT-6, a potent and selective inhibitor of the A2A adenosine receptor. Portage’s plan is to ultimately co-administer PORT-6 with PORT-7 in the ongoing ADPORT-601 trial. This will mark the first time two highly selective A2A and A2B antagonists are combined in patients, with the aim of achieving a complete blockade of adenosine-induced immunosuppression in the tumor microenvironment. This innovative approach is designed to fully neutralize adenosine-mediated immune suppression, enhance anti-tumor responses, and broaden the impact of immunotherapy in solid tumors. About Portage BiotechPortage Biotech is a clinical-stage immuno-oncology company advancing a pipeline of novel biologics to transform the immune system’s ability to fight cancer. For more information, visit www.portagebiotech.com. Forward-Looking StatementsAll statements in this news release, other than statements of historical facts, including without limitation, statements regarding the Company’s business strategy, plans and objectives of management for future operations and those statements preceded by, followed by or that otherwise include the words “believe,” “expects,” “anticipates,” “intends,” “estimates,” “will,” “may,” “plans,” “potential,” “continues,” or similar expressions or variations on such expressions are forward-looking statements. As a result, forward-looking statements are subject to certain risks and uncertainties, including, but not limited to: the risk that the Company may not secure financing, the uncertainty of the Company’s ability to continue as a going concern, scientific results may not be as expected, and other factors set forth in “Item 3 - Key Information-Risk Factors” in the Company’s Annual Report on Form 20-F for the year ended March 31, 2024 and “Business Environment – Risk Factors” in the Company’s Management’s Discussion and Analysis for the Three and Six Months ended September 30, 2024, filed as Exhibit 99.2 to the Company’s Form 6-K. Although the Company believes that the expectations reflected in these forward-looking statements are reasonable, undue reliance should not be placed on them as actual results may differ materially from these forward-looking statements. The forward-looking statements contained in this news release are made as of the date hereof, and the Company undertakes no obligation to update publicly or revise any forward-looking statements or information, except as required by law. For More Information:Portage BiotechAlexander Pickett, Chief Executive Officerir@portagebiotech.com
免疫疗法AACR会议临床研究
100 项与 University of L'Aquila 相关的药物交易
登录后查看更多信息
100 项与 University of L'Aquila 相关的转化医学
登录后查看更多信息
组织架构
使用我们的机构树数据加速您的研究。
登录
或

管线布局
2026年03月01日管线快照
管线布局中药物为当前组织机构及其子机构作为药物机构进行统计,早期临床1期并入临床1期,临床1/2期并入临床2期,临床2/3期并入临床3期
临床前
4
7
其他
登录后查看更多信息
当前项目
| 药物(靶点) | 适应症 | 全球最高研发状态 |
|---|---|---|
SI306-PD2 ( SRC family ) | 胶质母细胞瘤 更多 | 临床前 |
UniPR-1331 ( EphA2 ) | 实体瘤 更多 | 临床前 |
KPT-251 ( XPO1 ) | 前列腺癌 更多 | 临床前 |
KPT-185 ( XPO1 ) | 前列腺癌 更多 | 临床前 |
X-480 ( PI3Ks x mTOR ) | 骨转移癌 更多 | 无进展 |
登录后查看更多信息
药物交易
使用我们的药物交易数据加速您的研究。
登录
或

转化医学
使用我们的转化医学数据加速您的研究。
登录
或

营收
使用 Synapse 探索超过 36 万个组织的财务状况。
登录
或

科研基金(NIH)
访问超过 200 万项资助和基金信息,以提升您的研究之旅。
登录
或

投资
深入了解从初创企业到成熟企业的最新公司投资动态。
登录
或

融资
发掘融资趋势以验证和推进您的投资机会。
登录
或

生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用

