预约演示
更新于:2026-02-02

ReFlow Medical, Inc.
更新于:2026-02-02
概览
关联
8
项与 ReFlow Medical, Inc. 相关的临床试验NCT06122974
A RanDomized Controlled Trial of the Drug Eluting TEmporary Spur StEnt System vs PERcutaneous Balloon Angioplasty for the TReatment of CriTical Limb Ischemia
The purpose of this trial is to evaluate the safety and efficacy of the Drug Eluting Temporary Spur Stent System compared to PTA.
The Drug Eluting Temporary Spur Stent System is intended for use as a primary treatment in the infrapopliteal arteries for the treatment of de novo or restenotic lesions.
The Drug Eluting Temporary Spur Stent System is intended for use as a primary treatment in the infrapopliteal arteries for the treatment of de novo or restenotic lesions.
开始日期2026-08-01 |
申办/合作机构 |
NCT06117150
A Pilot Study of the Drug-eluting Coronary Spur StEnt as a Primary trEatment for In-stent Restenosis of the CORONARY Arteries (DEEPER CORONARY)
To demonstrate acceptable short term safety rates of the Drug-eluting Coronary Spur Stent System as a primary treatment for coronary in-stent restenosis.
开始日期2024-06-09 |
申办/合作机构 |
NCT05848232
A Prospective Single-Arm Multicenter Study Evaluating Use of the CORA Catheters for the Crossing of Coronary Chronic Total Occlusions
The goal of this clinical trial is to evaluate the safety and effectiveness of the coraFlex, coraForce, and coraCross catheters for crossing chronic total occlusions of the coronary arteries. The study will compare the rate of procedure success to success rates from previous trials.
Participants will undergo percutaneous coronary intervention (PCI) for a chronic total occlusion and be followed for 30 days post-procedure.
Participants will undergo percutaneous coronary intervention (PCI) for a chronic total occlusion and be followed for 30 days post-procedure.
开始日期2024-06-01 |
申办/合作机构 |
100 项与 ReFlow Medical, Inc. 相关的临床结果
登录后查看更多信息
0 项与 ReFlow Medical, Inc. 相关的专利(医药)
登录后查看更多信息
14
项与 ReFlow Medical, Inc. 相关的新闻(医药)2025-08-26
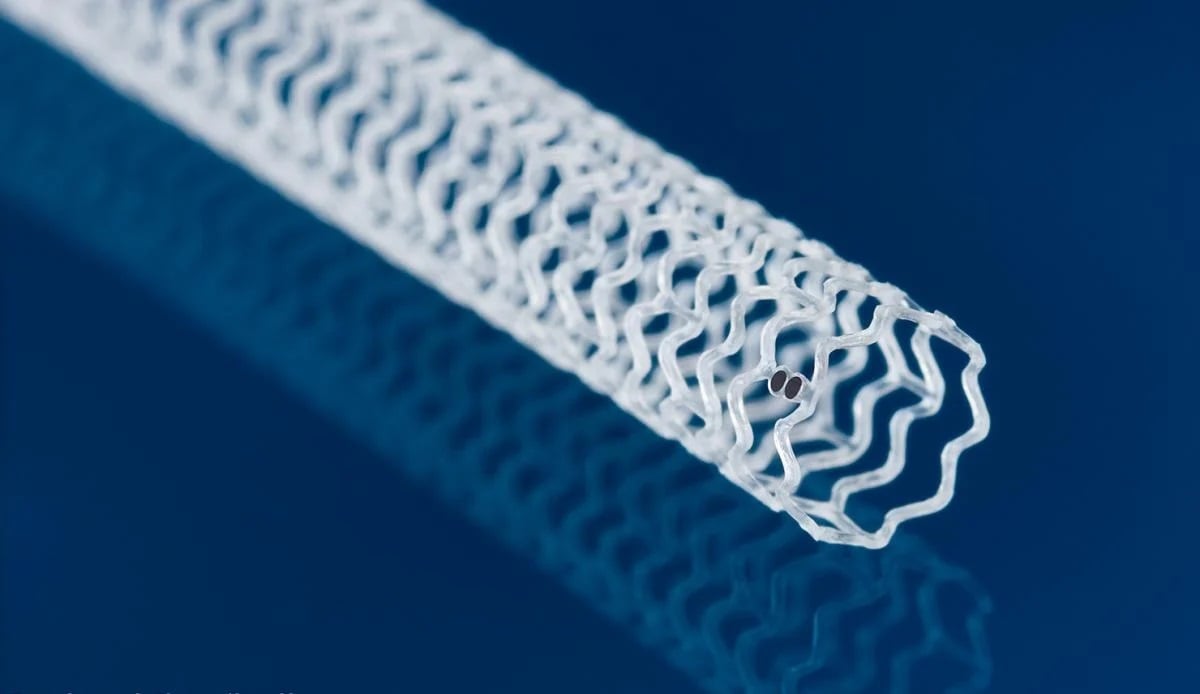
Made from a material akin to bioresorbable sutures, the temporary scaffold holds open blocked arteries while delivering a dose of the immunosuppressant everolimus, similar to Abbott\'s metal drug-eluting stents. \n Abbott has collected a green light in Europe for its dissolving, drug-laden stent in peripheral artery disease, following its approval from the FDA early last year. The company said the CE mark opens up the Esprit BTK scaffold treatment to more than 50 million people on the continent—about twice the population in the U.S.—including people with severe forms of the chronic disease who potentially face amputations of the lower legs and poor survival rates.Made from a material similar to bioresorbable sutures, the temporary stent holds open the blocked arteries located below the knee while delivering a dose of the immunosuppressant everolimus, which helps keep the vessel from reclosing as it heals. A previous clinical trial compared the Esprit BTK scaffold to the standard approach of balloon angioplasty, which showed that after one year, 74% of treated patients saw no amputations, completely blocked vessels or the need to redo the procedure, compared to just 44% of participants in the control arm. The stent is designed to be completely absorbed by the body after three years.Later findings from that study demonstrated that after two years, patients who received the Esprit BTK underwent about half as many procedures to reopen a closed artery versus balloon angioplasty alone. This particular field has not seen many technical advancements receive regulatory go-aheads until recently. Earlier this summer, the FDA granted a de novo clearance to a retrievable stent system for the lower limbs designed by Reflow Medical. The Spur carries a series of small, retractable spikes that puncture calcifications from within the arteries below-the-knee to help treat chronic limb-threatening ischemia. That device previously received a CE mark in January 2024.
上市批准临床结果AHA会议
2025-06-09
The agency’s de novo clearance in infrapopliteal arterial disease followed a successful clinical trial of patients who received below-the-knee treatments for chronic, limb-threatening ischemia. \n Reflow Medical has secured a groundbreaking green light from the FDA for its retrievable stent system, designed to boost blood flow through blockages in the lower limbs while leaving nothing behind. The company’s Spur implant carries a series of small spikes that penetrate calcifications from within the peripheral blood vessel, to help reduce the effects of recoil once the stent is removed from the artery. The device is designed to help treat long lesions, and can be used up to four times.The agency’s de novo clearance in infrapopliteal arterial disease followed a successful clinical trial of patients who received below-the-knee treatments for chronic, limb-threatening ischemia. The company said that after undergoing balloon dilation, the Spur self-expanding, bare-metal system saw a 99.2% successful placement and opening rate. After 30 days, 97.0% of patients reported no major adverse limb events, such as amputations, or perioperative deaths. “We are fully prepared to launch our innovative technology through our dedicated sales force, ensuring it promptly reaches physicians to support patients,” Reflow Medical’s co-founder and CEO, Isa Rizk, said in a statement. Earlier this year, Reflow Medical opened a European subsidiary in Germany, tasked with establishing direct sales channels and distribution partnerships on the continent. The company received a CE Mark approval for the Spur in January 2024.The company has also been developing a drug-eluting version of the Spur designed for use in the heart’s coronary arteries.

上市批准临床结果
2024-11-06
LAS VEGAS, Nov. 5, 2024 /PRNewswire/ -- The VIVA Foundation, a not-for-profit organization dedicated to advancing the field of vascular medicine and intervention through education and research, announces the results for the final two rounds of Late-Breaking Clinical Trials presented at the VIVA24 conference, at Wynn Las Vegas.
VIVA (Vascular InterVentional Advances) is an annual vascular education symposium that brings together a global, multispecialty faculty to present a variety of lectures and live case presentations from clinical centers around the world. The audience is composed of interventional cardiologists, interventional radiologists, vascular surgeons, and endovascular medicine specialists. Below are highlights of today's 8 late-breaking clinical trial presentations.
Two-Year Outcomes From the PROMISE II Trial of Transcatheter Arterialization of the Deep Veins
Presented by: Daniel Clair, MD
Up to 20% of patients with chronic limb-threatening ischemia (CLTI) are not eligible for conventional surgical or endovascular revascularization techniques and have a resultant amputation rate of 50% within 6 months. An alternative option for these no-option patients is transcatheter arterialization of the deep veins (TADV) with the purpose-built LimFlow System (Inari Medical), the only FDA-approved option. The LimFlow System consists of an integrated system for arteriovenous crossing, atraumatic vein preparation, and flow diversion. This work reports the 2-year outcomes from the prospective, multicenter, single-arm, PROMISE II trial of the LimFlow System for TADV in no-option patients.
All patients had Rutherford class 5/6 disease and were confirmed as ineligible for endovascular or surgical interventions by an independent physician review committee. Key exclusion criteria were systemic infection, rapidly deteriorating wounds, or advanced heart failure. Study conduct included an independent clinical events committee to adjudicate safety outcomes and a core laboratory to assess all wound images. Two-year outcomes included limb salvage, survival, amputation-free survival, wound healing, and Rutherford classification.
A total of 105 CLTI patients underwent TADV between 2018 and 2022. At 2 years, the limb salvage rate was 65%. An improvement was seen in Rutherford classification: 65.8% of patients had Rutherford class 4 or below and 54.3% had Rutherford class 0. Wounds were completely healed/healing in 82% of patients at 2 years; the mean wound area was 0.1 cm2, and the mean pain score was 1.2 out of 10. When combined with the PROMISE I trial, the 2-year limb salvage rate was 68%, with no differences observed based on age, sex, race, baseline Rutherford classification, diabetes, or dialysis.
The long-term outcomes from the PROMISE II trial represent data from the largest cohort of patients with 2-year data and demonstrate sustained limb salvage and wound healing in no-option CLTI patients.
Final Results of the DETOUR2 Study: Durability of Percutaneous Transmural Arterial Bypass for Treatment of Complex Femoropopliteal Disease
Presented by: Sean P. Lyden, MD
Percutaneous transmural arterial bypass (PTAB) with the DETOUR System (Endologix) is a novel endovascular procedure to treat complex femoropopliteal disease, including long lesions, heavy calcification, and chronic total occlusions (CTOs). The DETOUR System uses standard endovascular techniques with a unique crossing device and stent graft to create a percutaneous femoropopliteal bypass. We report on the final 3-year safety and effectiveness results of this innovative percutaneous revascularization.
The DETOUR2 investigational device exemption study is a prospective, single-arm, multicenter trial evaluating lesions > 20 cm in the femoropopliteal segment in patients with Rutherford class 3 to 5 disease across 36 sites. Follow-up visits were conducted at 30 days, 6 months, and annually through 3 years. The 36-month outcomes include clinically driven target lesion revascularization (CD-TLR) and primary patency, defined as the need for additional or secondary surgical or endovascular procedures. Venous events were also assessed.
Among the 202 patients enrolled and treated with the DETOUR System, 96% had CTOs. Mean lesion length was 327 ± 61 mm. Primary patency through 36 months was 58.2%. In DETOUR patients through 3 years, the freedom from CD-TLR rate for DETOUR patients was 66.8%; the major index limb amputation rate was 1.5%; and the all-cause mortality rate was 8.9%. Occurrence of deep vein thrombosis and pulmonary embolism through 3 years was unchanged from 2 years, at 4.1% and 0%, respectively. Overall Villalta scores and Venous Clinical Severity Score also remained unchanged from baseline through 3 years.
The 36-month outcomes from the DETOUR2 trial demonstrate the clinical utility and safety of this novel therapeutic strategy in complex femoropopliteal lesions. These long-term results suggest that PTAB with the DETOUR System achieves similar results to open surgical prosthetic femoropopliteal bypass. PTAB provides a standardized technique with durable outcomes when open surgical revascularization is not viable or traditional endovascular therapy has failed. Additional data from the PTAB1 postmarket, real-world registry will confirm the application of these findings to a wider population.
Retrievable Scaffold Therapy in Combination With a Paclitaxel-Coated Balloon: Two-Year Results of the DEEPER OUS Trial
Presented by: Thomas Zeller, MD
DEEPER OUS is a prospective, nonrandomized, multicenter, single-arm trial taking place in New Zealand, Germany, and Switzerland. The purpose of this trial is to evaluate the safety and efficacy of a novel device, the Spur Peripheral Retrievable Scaffold System (Reflow Medical, Inc.), in conjunction with a commercially available drug-coated balloon (DCB) in patients with symptomatic infrapopliteal arterial disease.
In-person follow-up occurred at 1, 3, 6, and 12 months postprocedure, with follow-up via telephone call annually out to 5 years.
The primary efficacy endpoint is primary patency of treated lesion sites by duplex ultrasound in patients free from clinically driven target lesion revascularization (CD-TLR) at 6 months. The primary safety endpoint is freedom from device- and procedure-related death through 30 days postprocedure. Secondary endpoints include safety-related and clinical outcome measures.
Lesion characteristics showed an average treated length of 92.7 ± 36.63 mm, and the most used Spur size was 3 X 60 mm, with an average reference vessel diameter of 3.1 ± 0.48 mm. Approximately 60% of vessels were mildly calcified, with the most common TASC classification being class B (37.2%, 51/106). The primary endpoints of primary patency at 6 months and freedom from perioperative death at 30 days were met and previously reported.
At 24 months, the secondary safety endpoint of freedom from major amputation of the target limb was met in 98.8% (79/80), all-cause mortality was 14% (15/107), and freedom from CD-TLR was 83.5% (71/85).
The Spur System is safe and effective for the treatment of infrapopliteal arterial disease when used in conjunction with a commercially available DCB.
How Sirolimus-Coated Balloon Angioplasty Compares to Various Paclitaxel-Coated Balloon Types–A Post Hoc Analysis of the Randomized SIRONA Trial
Presented by: Ulf Teichgräber, MD
The multicenter, randomized SIRONA trial provided a head-to-head comparison of sirolimus and paclitaxel drug-coated balloon (DCB) angioplasty for femoropopliteal lesions. However, the efficacy of paclitaxel DCB types varies considerably between vendors. Therefore, the purpose of this post hoc analysis was to determine whether the effectiveness of a sirolimus DCB (MagicTouch, Concept Medical) (n = 241) was dependent on the type of paclitaxel DCB used as a control.
The study enrolled 482 participants with Rutherford category 2 to 4 femoropopliteal artery disease. The mean lesion length was 84 ± 61 mm; 34% of lesions were totally occluded and 28% were severely calcified. The main subcontrol groups considered were Luminor 35 (iVascular) (n = 84), Lutonix (BD Interventional) (n = 46), Ranger (Boston Scientific Corporation) (n = 36), and In.Pact Admiral (Medtronic) (n = 36).
The primary efficacy endpoint was noninferiority in 12-month primary patency of sirolimus versus paclitaxel DCB and was met: 73.8% versus 75% (rate difference of -1.2%; 95% CI, -9.97% to 7.4%; P = .022). The odds ratio for binary restenosis did not differ significantly between study groups, regardless of the type of paclitaxel DCB used. There was no significant difference in 12-month freedom from clinically driven target lesion revascularization between sirolimus and paclitaxel DCB, regardless of the paclitaxel DCB used. The primary composite safety noninferiority endpoint for the sirolimus DCB was met.
Given that sirolimus has a wider therapeutic window than paclitaxel, it may serve as a welcome alternative for femoropopliteal DCB angioplasty.
Impact of the Global Limb Anatomic Staging System (GLASS) on Clinical Outcomes in the LIFE-BTK Randomized Controlled Trial
Presented by: Brian G. DeRubertis, MD
The LIFE-BTK trial investigated outcomes in patients with chronic limb-threatening ischemia (CLTI) due to infrapopliteal peripheral artery disease treated with percutaneous transluminal angioplasty (PTA). Authors compared the use of the Esprit BTK Drug-Eluting Resorbable Scaffold (Abbott) with PTA, focusing on the Global Limb Anatomic Staging System (GLASS), which identifies different anatomic patterns of disease. The study included 261 patients randomized in a 2:1 ratio to receive either the Esprit BTK or PTA. The primary efficacy endpoint—the composite of freedom from above-ankle amputation, target vessel occlusion, target lesion binary restenosis, and clinically driven target lesion revascularization at 1 year—freedom from events occurred in 72.1% of patients in the GLASS stage I group compared to 53.3% in the GLASS II to III group. This 18.78% difference was statistically significant (P = .0068) (see freedom from Kaplan-Meier rates in poster). Additionally, the freedom from target vessel occlusion and binary restenosis were consistent with the primary endpoint, both favoring the GLASS stage I group. Furthermore, patients treated with the Esprit BTK scaffold demonstrated improved outcomes, regardless of GLASS classification, when compared to those receiving PTA.
In conclusion, the GLASS system effectively identified patients at higher risk for adverse clinical events. The use of Esprit BTK in CLTI patients showed consistent results irrespective of GLASS staging.
Diversity, Equity, and Inclusion in the LIFE-BTK Trial Evaluating the Esprit BTK Drug-Eluting Resorbable Scaffold for Treatment of Infrapopliteal Lesions in Patients With CLTI
Presented by: Lawrence A. Garcia, MD
In the LIFE-BTK trial, several initiatives were implemented to facilitate enrollment of diverse populations, ensuring that patients most affected by the disease were represented. Initiatives geared toward the patients and research staff at the sites included: (1) a study website providing information on clinical trials, disease state, and the LIFE-BTK trial; (2) patient and site brochures with information on disease state and the trial; (3) translation services in > 48 languages with interpreters available 24/7; (4) patient reimbursement for travel and assistance in booking airfare and hotel; and (5) option to conduct follow-up visits in the patient's home. The primary effectiveness endpoint, as well as the two powered secondary endpoints, were evaluated based on various groupings of race and ethnicity.
LIFE-BTK enrolled a total of 261 patients in the United States, Australia, New Zealand, Taiwan, Hong Kong, and Singapore, of which 12.3% identified as Black or African American, 18% as Asian, 59% as White, and 16.5% as Hispanic. The trial met its primary effectiveness endpoint of limb salvage and primary patency at 1 year, demonstrating superiority of Esprit BTK Drug-Eluting Resorbable Scaffold (Abbott) over percutaneous transluminal angioplasty. The primary safety endpoint was also met. Analysis of the primary effectiveness endpoint in various race/ethnicity groupings showed consistent results with the overall population, with risk ratios between 0.32 and 0.62 in favor of Esprit BTK compared to a risk ratio of 0.45 in the overall population. The same trend was observed for both powered secondary endpoints, with risk ratios ranging from 0.32 to 0.68 and 0.30 to 0.72 for the first and second powered secondary endpoints, respectively.
The LIFE-BTK trial enrolled a patient population whose race distribution was comparable to Centers for Medicare & Medicaid Services patients with a diagnosis of chronic limb-threatening ischemia undergoing endovascular procedures. Primary effectiveness and powered secondary endpoint results in the various race and ethnicity subgroups analyzed were consistent with the overall population as to outcome with the Esprit BTK device. LIFE-BTK's focus on ethnic and racial diversity was an important and successful goal.
STRIDE Study Suggests Race, Not Sex, Is Associated With Outcomes in Acute Limb Ischemia Treated With Mechanical Aspiration Thrombectomy
Presented by: Alex Powell, MD
STRIDE assessed safety, efficacy, and quality of life (QoL) outcomes through 365 days for lower extremity acute limb ischemia (LE-ALI) patients treated first line with mechanical aspiration thrombectomy using the Indigo Aspiration System (Penumbra, Inc.). STRIDE was a prospective, single-arm study that enrolled 119 patients across 16 sites (United States and Europe).
The primary outcome was target limb salvage (TLS) at 30 days postprocedure. Secondary outcomes included device-related serious adverse events, technical success (postprocedure thrombolysis in myocardial infarction flow grades 2/3), mortality, and 30-day patency. In these subgroup analyses, sex differences were analyzed, and a multivariate Cox proportional hazards model was developed to identify predictors of time to primary and secondary outcomes.
For all patients (mean age, 66.3 years; 46.2% female; 20.2% African American), TLS and patency at 30 days were 98.2% (109/111) and 89.4% (101/113), respectively. No sex differences were observed for 30-day TLS (P > .999), 30-day patency (P = .220), 365-day mortality (P = .223), or change in QoL (P = .372). Periprocedural major bleeding (not device related) occurred at a higher rate in females (9.1% vs 0%; P = .019), but all had below-normal range of preprocedural hemoglobin and hematocrit levels or a chronic history of anemia. In the multivariate model, race and sex were not associated with 30-day patency or 365-day mortality. However, African American patients had increased risk of limb loss (hazard ratio, 13.2; 95% CI, 2.5-68.6) and a reduced QoL improvement at 365 days (∆ -3.1; 95% CI, -6.7 to 0.4).
Data from STRIDE continue to support Indigo as a safe and effective option for first-line treatment of LE-ALI in a diverse patient population. Males and females experienced good efficacy, safety, and improved QoL outcomes, despite literature reporting females having a history of poorer outcomes than males. Multivariate analyses identified increased risks for African American patients, underscoring the need for continued research into underlying baseline risk factors and efforts to achieve equity in LE-ALI treatment.
Endovascular PAD Treatment With Drug-Eluting Devices in a Registry Focused on Underrepresented Minorities and Women: 12-Month Clinical Results From the First 500 ELEGANCE Registry Patients
Presented by: Jay Giri, MD, MPH
The ELEGANCE registry examines endovascular drug-eluting peripheral artery disease (PAD) treatment with a focus on historically underrepresented patient populations. Study participants underwent peripheral drug-eluting device interventions with the Ranger Paclitaxel-Coated Balloon and/or Eluvia Paclitaxel-Eluting Stent (both Boston Scientific Corporation). Through novel recruitment mechanisms, the study aims to enroll at least 40% women and 40% underrepresented racial and ethnic groups globally.
Characteristics of the first 500 patients who completed a 12-month follow-up visit aligned with ELEGANCE enrollment objectives, with 41.6% female patients and 41.6% from underrepresented racial/ethnic populations. Patient characteristics and disease presentation differed among sex and race/ethnicity groups. Females were significantly older than males on average and more likely to present with advanced Rutherford category 4 to 6 disease. All underrepresented population groups presented as Rutherford category 4 to 6 at a greater frequency than the non-Hispanic White group.
Despite differing and advanced disease characteristics, the 12-month above-ankle amputation incidence was low at 0.8%; reintervention rates were low and did not differ significantly by sex or race and ethnicity. The overall site-reported 12-month freedom from clinically driven target lesion revascularization rate was 89.6%.
Although the PAD presentation of the diverse patients enrolled in ELEGANCE reflects significant disparities, 1-year outcomes of paclitaxel-based revascularization with the Ranger Drug-Coated Balloon or Eluvia Drug-Eluting Stent suggest that excellent effectiveness and safety results may generalize to underrepresented patient groups.
About the VIVA Foundation
The VIVA Foundation, a not-for-profit organization dedicated to advancing the field of vascular medicine and intervention through education and research, strives to be the premier educator in the field. Our team of specialists in vascular medicine, interventional cardiology, interventional radiology, and vascular surgery is driven by the passion to advance the field and improve patient outcomes. Educational events presented by the VIVA Foundation have a distinct spirit of collegiality attained by synergizing collective talents to promote awareness and innovative therapeutic options for vascular disease worldwide.
To learn more about the VIVA Foundation, visit .
SOURCE The VIVA Foundation
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
440k+
Newsrooms &
Influencers
9k+
Digital Media
Outlets
270k+
Journalists
Opted In
GET STARTED

临床结果AHA会议临床1期临床2期
100 项与 ReFlow Medical, Inc. 相关的药物交易
登录后查看更多信息
100 项与 ReFlow Medical, Inc. 相关的转化医学
登录后查看更多信息
组织架构
使用我们的机构树数据加速您的研究。
登录
或

管线布局
2026年02月08日管线快照
无数据报导
登录后保持更新
药物交易
使用我们的药物交易数据加速您的研究。
登录
或

转化医学
使用我们的转化医学数据加速您的研究。
登录
或

营收
使用 Synapse 探索超过 36 万个组织的财务状况。
登录
或

科研基金(NIH)
访问超过 200 万项资助和基金信息,以提升您的研究之旅。
登录
或

投资
深入了解从初创企业到成熟企业的最新公司投资动态。
登录
或

融资
发掘融资趋势以验证和推进您的投资机会。
登录
或

生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用