预约演示
更新于:2025-05-07
Strides Pharma (Cyprus) Ltd.
子公司|Cyprus
子公司|Cyprus
更新于:2025-05-07
概览
标签
内分泌与代谢疾病
皮肤和肌肉骨骼疾病
其他疾病
重组多肽
生物类似药
疾病领域得分
一眼洞穿机构专注的疾病领域
暂无数据
技术平台
公司药物应用最多的技术
暂无数据
靶点
公司最常开发的靶点
暂无数据
| 疾病领域 | 数量 |
|---|---|
| 内分泌与代谢疾病 | 1 |
| 排名前五的药物类型 | 数量 |
|---|---|
| 生物类似药 | 1 |
| 重组多肽 | 1 |
| 排名前五的靶点 | 数量 |
|---|---|
| PTH1R(甲状旁腺激素受体) | 1 |
关联
1
项与 Strides Pharma (Cyprus) Ltd. 相关的药物1
项与 Strides Pharma (Cyprus) Ltd. 相关的临床试验PACTR201303000491420
Contraceptive and safety studies of UniPron
开始日期2013-04-30 |
申办/合作机构  Universal Corp. Ltd. Universal Corp. Ltd. [+2] |
100 项与 Strides Pharma (Cyprus) Ltd. 相关的临床结果
登录后查看更多信息
0 项与 Strides Pharma (Cyprus) Ltd. 相关的专利(医药)
登录后查看更多信息
6
项与 Strides Pharma (Cyprus) Ltd. 相关的新闻(医药)2025-03-12
DUBAI, UAE, March 12, 2025 /PRNewswire/ -- XRP Healthcare, a pioneering force in healthcare innovation, has officially acquired Pharma Ville, a leading retail and wholesale pharmacy chain in Uganda.
This strategic acquisition marks XRP Healthcare's entry into Africa's rapidly evolving healthcare sector, laying the foundation for scalable, technology-driven pharmaceutical distribution across the continent.
Continue Reading
XRP Healthcare Acquires Pharma Ville, a Retail and Wholesale Pharmacy Chain in Uganda
XRP Healthcare Acquires Pharma Ville, a Retail and Wholesale Pharmacy Chain in Uganda
With two retail pharmacies and five wholesale distribution centres strategically positioned across Uganda, Pharma Ville's established infrastructure enhances medicine accessibility nationwide. The acquisition strengthens XRP Healthcare's ability to streamline pharmaceutical supply chains and expand its footprint in Africa's healthcare market.
Shonubi Musoke & Co. Advocates, one of Uganda's premier law firms and a partner of the globally renowned Norton Rose Fulbright, advised XRP Healthcare in this transaction, ensuring a seamless legal and regulatory transition.
Pharma Ville plays a pivotal role in Uganda's healthcare sector, working closely with the National Medical Stores (NMS) and Joint Medical Stores (JMS) to ensure a steady and reliable supply of essential medicines.
The acquisition includes 60 registered pharmaceutical products, 18 products ready for immediate commercialization, and 70 additional products in the pipeline awaiting regulatory approval.
Additionally, Pharma Ville has distribution agreements with eight global pharmaceutical suppliers, reinforcing its strong supply chain network:
Toros Group (Switzerland) – Orthopedic supplies
Ascensia Switzerland (Germany) – Diabetic care solutions
Incepta Pharmaceuticals Ltd (Bangladesh) – Human drugs
Amanta Health Care Limited (India) – Intravenous fluids
Forans Latvia (Latvia) – Diabetic care products
Mediteks (Turkey) – Surgical equipment
Naari Pharma Pvt Limited (India) – Human drugs
Universal Corporation Limited (Kenya) – Human drugs
Richard Kitonsa (MPS), Founder and CEO of Pharma Ville, emphasized the transformative impact of this acquisition:
"Joining XRP Healthcare is transformative. We can now rapidly scale our operations, introduce innovative technologies, and better serve Uganda's healthcare needs."
Whitney Lynn, Chairman of XRP Healthcare, underscored the company's mission:
"Our goal is simple—make essential medicines more accessible and affordable. This acquisition is a major step toward achieving that."
Kain Roomes, CEO of XRP Healthcare, highlighted the opportunity within Uganda's healthcare sector:
"Uganda's fragmented pharmaceutical landscape presents immense potential. By integrating Pharma Ville, we can deliver immediate impact—improving access to essential medicines and optimizing healthcare delivery."
Laban Roomes, COO of XRP Healthcare, emphasized the groundwork laid for expansion:
"We have spent significant time in Uganda developing the legal and operational frameworks necessary to scale this business rapidly. Our efforts will drive long-term improvements in healthcare accessibility."
Pharma Ville will undergo immediate operational upgrades, including:
Advanced inventory management to optimize supply chains
Upgraded licensing to expand product offerings
Enhanced distribution channels for wider reach
Digital payment integration for seamless transactions
New CRM systems to improve customer engagement
By Q4, Pharma Ville will rebrand as XRP Healthcare, aligning with the company's vision for innovation and healthcare excellence.
The XRPH AI app, a multilingual healthcare assistant, is already live and integrated into Pharma Ville's digital platform (). The AI-driven tool provides medical guidance in Luganda, Swahili, Kinyarwanda, French, and English.
In an upcoming update, users will be able to upload images of medical conditions for AI-assisted preliminary assessments, with direct referrals to healthcare professionals for critical cases.
Peter Waiswa, a strategic advisor to UNICEF, the Bill & Melinda Gates Foundation, and now XRP Healthcare, praised the acquisition's significance:
"This acquisition strengthens Uganda's healthcare sector, fosters economic growth, and introduces essential technologies to the industry. It's a major step forward for healthcare accessibility in Uganda."
With Africa's healthcare market projected to reach $259 billion by 2030, and the digital health segment—encompassing AI-driven solutions—expected to grow to $16.6 billion, XRP Healthcare is strategically positioned to capitalize on these transformative opportunities.
XRP Healthcare holds registered trademarks in Uganda, the UAE, and the UK, with ongoing global registrations, reinforcing its commitment to innovation and brand excellence.
Additionally, XRP Healthcare has informed its community of an upcoming global announcement, separate from its African expansion, to be revealed this quarter. This development reflects the company's broader strategic ambitions and commitment to advancing global healthcare solutions.
About XRP Healthcare
XRP Healthcare is a pioneering healthcare solutions provider leveraging AI and strategic acquisitions to enhance healthcare accessibility and efficiency. By integrating digital innovation with traditional pharmaceutical distribution, XRP Healthcare is at the forefront of revolutionizing healthcare delivery in emerging markets.
Photo -
Photo -
Logo -
SOURCE XRP Healthcare
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
440k+
Newsrooms &
Influencers
9k+
Digital Media
Outlets
270k+
Journalists
Opted In
GET STARTED
并购
2025-03-05
TOKYO, March 4, 2025 /PRNewswire/ -- The Global Health Innovative Technology (GHIT) Fund is today announcing a significant milestone: the first administration of arpraziquantel to preschool-aged children in Uganda, in an implementation science setting. This new pediatric treatment for schistosomiasis, developed by the GHIT-funded Pediatric Praziquantel Consortium, is being introduced through the Consortium's ADOPT program, which focuses on integrating arpraziquantel into existing healthcare platforms in countries where schistosomiasis is prevalent. This achievement marks GHIT's first supported innovation to reach people in need since its establishment in 2013.
"This isn't just a scientific achievement," said Osamu Kunii, CEO of the GHIT Fund. "It's a testament to the transformative power of global collaboration. By bringing together a diverse array of partners, each steadfastly contributing their unique expertise and sustaining momentum throughout a decade-long marathon of innovation to shepherd this breakthrough (from bench to bedside), arpraziquantel's development journey has unlocked solutions that once seemed impossible."
Schistosomiasis, or bilharzia, affects 250 million people globally, including an estimated 50 million preschool-aged children, mainly in sub-Saharan Africa. Left untreated, this poverty-related parasitic disease can lead to anemia, stunted growth, and impaired learning ability, as well as chronic inflammation of the organs, which can be fatal. Until now, a child-friendly treatment specifically tailored for preschool-aged children was not available, leaving millions of preschoolers at risk.
Early treatment of schistosomiasis in preschool-aged children is a crucial investment in public health and societal wellbeing. By intervening at this critical stage, we not only aim to shield young bodies from severe complications like organ damage and cognitive impairment but also alleviate the long-term burden on healthcare systems and hopefully better livelihoods.
The Pediatric Praziquantel Consortium developed arpraziquantel to address this gap. Japan's Astellas Pharma Inc., as a founding member of the Pediatric Praziquantel Consortium, played a pivotal role by utilizing its proprietary technology to lead arpraziquantel's initial formulation development, resulting in water dispersible, climate-stable, child-friendly tablets with acceptable taste. The formulation was optimized by Merck in Germany; the manufacturing process served to produce clinical trial supply from Merck and Farmanguinhos in Brazil. Current manufacturing is done by Farmanguinhos; future large-scale production by Universal Corporation Ltd. in Kenya is planned in and for Africa.
"Japanese innovation is a cornerstone of arpraziquantel's development," Dr. Kunii added, "embodying GHIT Fund's core mission: harnessing the power of international partnerships to revolutionize global health."
GHIT Fund has supported arpraziquantel's development journey throughout the overall development program spanning over 10 years. GHIT has and continues to support the Pediatric Praziquantel Consortium in developing their access plans, with a particular focus on exploring new and sustainable access mechanisms. GHIT is also co-funding, with EDCTP, implementation via the Consortium's ADOPT Program, which is assessing different implementation platforms in three countries: Côte d'Ivoire, Uganda and Kenya. In addition, GHIT is coordinating, together with the consortium, in-country readiness preparations for introduction of arpraziquantel in additional countries such as Senegal and Tanzania.
"Understanding what needs to be in place at country level in terms of policy framework, regulatory framework, implementation models and domestic resource mobilization is critical to the successful uptake and roll out of arpraziquantel. We hope the lessons learnt from these countries will inform other countries," said Dr Isaac Chikwanha, Senior Director of Access at the GHIT Fund.
Arpraziquantel received European Medicines Agency's positive scientific opinion in December 2023 and was included into the World Health Organization (WHO)'s List of Prequalified Medicines in May 2024. Inclusion in the WHO's Essential Medicines List is expected in 2025.
1.
About schistosomiasis
Schistosomiasis (also known as bilharzia) is one of the most prevalent parasitic diseases worldwide and a very important one in terms of public health burden and economic impact. It is a poverty-related disease that is widespread in tropical and subtropical regions where large sections of the population have no access to clean water. Flatworms transmit the disease and people become infected with the parasite through contact with freshwater, for example, while working, swimming, fishing, or washing their clothes. The minuscule larvae penetrate human skin, enter the blood vessels, and attack internal organs. The infection rate is particularly high among children. Schistosomiasis is a chronic condition and is classified by the World Health Organization (WHO) as one of the 21 neglected tropical diseases (NTDs).
2.
About arpraziquantel
The current standard of care treatment for schistosomiasis is praziquantel. Praziquantel is already approved, and suitable for school-aged children and adults. Extending the range of options for the treatment of schistosomiasis, arpraziquantel is tailored for preschool-aged children against Schistosoma mansoni and Schistosoma haematobium. Tested in clinical development, under the responsibility of Merck, arpraziquantel is a 150mg dispersible tablet. The prototype of its pediatric formulation was developed by Astellas Pharma Inc. in Japan, and further optimized by Merck in Germany.
In developing arpraziquantel, the Pediatric Praziquantel Consortium established a pediatric drug development program, divided into four major steps: preclinical development, clinical development, registration, and access. All details can be found on the Consortium website.
3.
About the Pediatric Praziquantel Consortium
The Pediatric Praziquantel Consortium is an international public-private partnership that aims to reduce the global disease burden of schistosomiasis and improve child health by addressing the medical needs of preschool-aged children. Its mission has been to develop, register, and provide access to a suitable pediatric drug for treating schistosomiasis in children 3 months to 6 years of age. For more information, and to see an overview of all Consortium partners, visit the Consortium website:
The GHIT Fund is a Japan-based international public-private partnership (PPP) fund that was formed between the Government of Japan, multiple pharmaceutical companies, the Bill & Melinda Gates Foundation, Wellcome, and the United Nations Development Programme (UNDP). The GHIT Fund invests in and manages an R&D portfolio of development partnerships aimed at addressing neglected diseases, such as malaria, tuberculosis, and neglected tropical diseases, which afflict the world's vulnerable and underserved populations. In collaboration with global partners, the GHIT Fund mobilizes Japanese industry, academia, and research institutes to create new drugs, vaccines, and diagnostics for malaria, tuberculosis, and neglected tropical diseases.
For more information, contact:
Katy Lenard at +1-301-280-5719 or [email protected]
Eriko Mugitani at +81-36441-2032 or [email protected]
SOURCE GHIT Fund
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
440k+
Newsrooms &
Influencers
9k+
Digital Media
Outlets
270k+
Journalists
Opted In
GET STARTED
2023-12-17
- Astellas contributed to the development of a pediatric formulation to treat schistosomiasis as a member of the Pediatric Praziquantel Consortium -
TOKYO, Dec. 17, 2023 /PRNewswire/ -- Astellas Pharma Inc. (TSE: 4503, President and CEO: Naoki Okamura, "Astellas") contributed to the development of a pediatric formulation to treat schistosomiasis as a member of the Pediatric Praziquantel Consortium (Consortium). The Consortium announced that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive scientific opinion for arpraziquantel to treat one of the neglected tropical diseases, schistosomiasis, in preschool-aged children (3 months to 6 years of age) on December 15. EMA assessed arpraziquantel under the EU-Medicines for all (EU-M4all) *1 procedure for high-priority medicines intended for use in countries outside the European Union. For more details on arpraziquantel, please refer to the Consortium's press release.
Continue Reading
Astellas provided its innovative formulation technology to co-develop arpraziquantel as a member of the Consortium. The new pediatric formulation of arpraziquantel is palatable for very young children by reducing the bitter taste associated with the medication and administered via a 150 mg dispersible tablet that is dissolved in water. The prototype of the pediatric formulation has been developed by Astellas and further optimized by Merck (Germany) for global production using simple manufacturing processes yielding tablets that can remain shelf stable, even in hot and humid tropical climates. Farmanguinhos (Brazil) brings expertise in production and distribution and will be the manufacturing site for the future introduction of the new pediatric medication in endemic countries. The partnership with Universal Corporation Ltd. (Kenya) is also supporting the planned future large-scale local production to serve African countries.
In parallel with this regulatory work, the Consortium's implementation research program (ADOPT)*2 is ongoing and preparing for the introduction of arpraziquantel in the first endemic countries in Africa. To ensure equitable and sustainable access, it is essential that new procurement and funding mechanisms are collaboratively explored and established. The intent is to make the product available at an at-cost basis in sub-Saharan African countries.
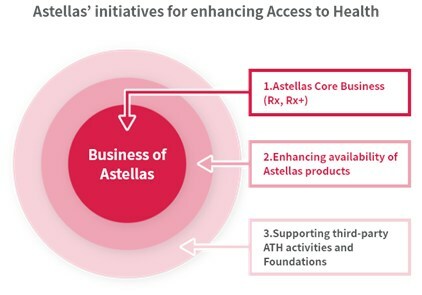
Astellas has set a Strategic Goal to "deepen our engagement in sustainability" in the Astellas Corporate Strategic Plan 2021. To address the key sustainability issue of Access to Health (ATH), Astellas uses a comprehensive approach including, (1) Astellas' core business (Rx, Rx+), (2) Enhancing availability of Astellas products (Access to Medicines), and (3) Collaboration and support for the activities implemented by external partners. Initiatives in the Consortium apply to (3), and the positive CHMP scientific opinion by EMA of arpraziquantel represents a significant milestone in alignment with Astellas' Access to Health focus.
Naoki Okamura, President and CEO, Astellas
"We are so pleased that arpraziquantel received a positive scientific opinion by EMA, and also proud that Astellas could contribute to its development by providing our innovative formulation technologies. Access to Health is essential to the health of people around the world, and we recognize it as a material issue for Astellas. Through our VISION to be "On the forefront of healthcare change to turn innovative science into VALUE for patients", we are proactively taking a comprehensive approach to solving this issue."
For more information on Astellas' contribution to the development of arpraziquantel, please see ().
About schistosomiasis
Schistosomiasis (also known as bilharzia) is one of the most prevalent parasitic diseases worldwide and a very important one in terms of public health burden and economic impact. It is a poverty-related disease that is widespread in tropical and subtropical regions where large sections of the population have no access to clean water. Flatworms transmit the disease and people become infected with the parasite through contact with freshwater, for example, while working, swimming, fishing, or washing their clothes. The minuscule larvae penetrate human skin, enter the blood vessels, and attack internal organs. The infection rate is particularly high among children. Schistosomiasis is a chronic condition and is classified by the World Health Organization (WHO) as one of 20 neglected tropical diseases (NTDs).
About Arpraziquantel
The current standard of care treatment for schistosomiasis is praziquantel. Praziquantel is safe, effective, and suitable for school-aged children and adults. Extending the range of options for the treatment of schistosomiasis, arpraziquantel is tailored for preschool-aged children against Schistosoma mansoni and Schistosoma haematobium. Tested in clinical development, under the responsibility of Merck, arpraziquantel contains the pharmacologically active enantiomer of praziquantel. In developing arpraziquantel, the Pediatric Praziquantel Consortium established a pediatric drug development program, divided into four major steps: preclinical development, clinical development, registration, and access. All details can be found on the Consortium website.
About the Pediatric Praziquantel Consortium
The Pediatric Praziquantel Consortium is an international public-private partnership that aims to reduce the global disease burden of schistosomiasis and improve child health by addressing the medical needs of infected preschool-aged children. Its mission is to develop, register, and provide access to a suitable pediatric drug for treating schistosomiasis in children 3 months to 6 years of age. For more information, and to see an overview of all Consortium partners, visit the Consortium website.
Consortium Partners
Merck (Germany)
Astellas Pharma Inc. (Japan)
The Swiss Tropical and Public Health Institute (Switzerland)
Lygature (The Netherlands)
Farmanguinhos (Brazil)
Unlimit Health (United Kingdom)
Kenya Medical Research Institute (Kenya)
Université Félix Houphouët-Boigny (Côte d'Ivoire)
Klinikum rechts der Isar der Technischen Universität München (Germany)
Ministry of Health Côte d'Ivoire (Côte d'Ivoire)
African Institute for Health and Development (Kenya)
Other collaborators that contribute to the mission of the Pediatric Praziquantel Consortium:
Makerere University School of Public Health (Uganda)
Ministry of Health Kenya, Division of Vector Borne and NTDs (Kenya)
Ministry of Health Uganda, Vector Borne and NTDs Control Division (Uganda)
About Astellas
Astellas Pharma Inc. is a pharmaceutical company conducting business in more than 70 countries around the world. We are promoting the Focus Area Approach that is designed to identify opportunities for the continuous creation of new drugs to address diseases with high unmet medical needs by focusing on Biology and Modality. Furthermore, we are also looking beyond our foundational Rx focus to create Rx+® healthcare solutions that combine our expertise and knowledge with cutting-edge technology in different fields of external partners. Through these efforts, Astellas stands on the forefront of healthcare change to turn innovative science into VALUE for patients. For more information, please visit our website at .
Cautionary Notes
In this press release, statements made with respect to current plans, estimates, strategies and beliefs and other statements that are not historical facts are forward-looking statements about the future performance of Astellas. These statements are based on management's current assumptions and beliefs in light of the information currently available to it and involve known and unknown risks and uncertainties. A number of factors could cause actual results to differ materially from those discussed in the forward-looking statements. Such factors include, but are not limited to: (i) changes in general economic conditions and in laws and regulations, relating to pharmaceutical markets, (ii) currency exchange rate fluctuations, (iii) delays in new product launches, (iv) the inability of Astellas to market existing and new products effectively, (v) the inability of Astellas to continue to effectively research and develop products accepted by customers in highly competitive markets, and (vi) infringements of Astellas' intellectual property rights by third parties.
Information about pharmaceutical products (including products currently in development) which is included in this press release is not intended to constitute an advertisement or medical advice.
SOURCE Astellas Pharma Inc.
上市批准
100 项与 Strides Pharma (Cyprus) Ltd. 相关的药物交易
登录后查看更多信息
100 项与 Strides Pharma (Cyprus) Ltd. 相关的转化医学
登录后查看更多信息
组织架构
使用我们的机构树数据加速您的研究。
登录
或

管线布局
2026年02月08日管线快照
管线布局中药物为当前组织机构及其子机构作为药物机构进行统计,早期临床1期并入临床1期,临床1/2期并入临床2期,临床2/3期并入临床3期
批准上市
1
登录后查看更多信息
当前项目
| 药物(靶点) | 适应症 | 全球最高研发状态 |
|---|---|---|
特立帕肽(Strides Pharma (Cyprus)) ( PTH1R ) | 糖皮质激素诱导的骨质疏松症 更多 | 批准上市 |
登录后查看更多信息
药物交易
使用我们的药物交易数据加速您的研究。
登录
或

转化医学
使用我们的转化医学数据加速您的研究。
登录
或

营收
使用 Synapse 探索超过 36 万个组织的财务状况。
登录
或

科研基金(NIH)
访问超过 200 万项资助和基金信息,以提升您的研究之旅。
登录
或

投资
深入了解从初创企业到成熟企业的最新公司投资动态。
登录
或

融资
发掘融资趋势以验证和推进您的投资机会。
登录
或

生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用