预约演示
更新于:2025-09-05

Biomap (Beijing) Intelligent Technology Co., Ltd.
更新于:2025-09-05
概览
标签
肿瘤
免疫系统疾病
消化系统疾病
抗体
重组多肽
疾病领域得分
一眼洞穿机构专注的疾病领域
暂无数据
技术平台
公司药物应用最多的技术
暂无数据
靶点
公司最常开发的靶点
暂无数据
| 排名前五的药物类型 | 数量 |
|---|---|
| 抗体 | 11 |
| 未知 | 3 |
| 重组多肽 | 1 |
| 排名前五的靶点 | 数量 |
|---|---|
| GLP-2R(胰高血糖素样肽2受体) | 1 |
关联
15
项与 百图生科(北京)智能技术有限公司 相关的药物靶点- |
作用机制- |
在研机构 |
原研机构 |
在研适应症 |
非在研适应症- |
最高研发阶段临床前 |
首次获批国家/地区- |
首次获批日期- |
靶点 |
作用机制 GLP-2R激动剂 |
在研机构 |
原研机构 |
在研适应症- |
非在研适应症- |
最高研发阶段临床前 |
首次获批国家/地区- |
首次获批日期- |
靶点- |
作用机制- |
在研机构 |
原研机构 |
在研适应症 |
非在研适应症- |
最高研发阶段临床前 |
首次获批国家/地区- |
首次获批日期- |
100 项与 百图生科(北京)智能技术有限公司 相关的临床结果
登录后查看更多信息
0 项与 百图生科(北京)智能技术有限公司 相关的专利(医药)
登录后查看更多信息
129
项与 百图生科(北京)智能技术有限公司 相关的新闻(医药)2025-08-23
Proprietary sequence-first model rivals and in key areas surpasses the leading ProteinGym sequence-only baselines
Sequence is the new paradigm”
— Dr. Lurong Pan
SAN FRANCISCO, CA, UNITED STATES, August 23, 2025 /
EINPresswire.com
/ --
Ainnocence
, a pioneer in generative AI for biotechnology, today announced the successful internal benchmarking of its protein language model, AINN-P1, trained on UniRef sequences with up to 167 million parameters.
AINN-P1 is Ainnocence’s protein language foundation model, built to understand protein sequence patterns to power next-generation drug discovery. The new model posts state-of-the-art Spearman correlation scores across four core protein-fitness tasks, reinforcing Ainnocence’s sequence-first drug-discovery platform and underscoring its competitiveness against much larger transformer models from Meta and BioMap.
Highlights:
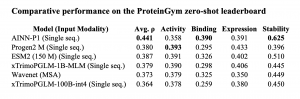
• Top overall sequence-only score: With an average ρ = 0.441, AINN-P1 edges past ProSST (0.438) while using orders-of-magnitude fewer parameters than 100 B-scale transformers.
• Best-in-class stability prediction: A ρ = 0.625 on stability is the highest of any published sequence-first model, providing critical accuracy for manufacturability screens.
• Closing the binding gap: AINN-P1’s binding ρ = 0.390 exceeds ESM2-150M by 0.064 points, narrowing the historic advantage of structure-aware models.
Detailed benchmark results
Recent in-house evaluations of the AINN-P1 performed in a few-shot embedding regime for efficiency, produced the following Spearman correlations: Activity 0.3581, Binding 0.3901, Expression 0.3913, Stability 0.6251. These metrics match or exceed many transformer-based benchmarks, demonstrating that smart architecture and curated data can beat brute-force scale.
Crucially, these benchmarks were achieved without expensive full fine-tuning. Instead, fixed AINN-P1 embeddings feed lightweight regressors – a strategy that slashes compute costs while preserving accuracy, and aligns with emerging best practices when only limited experimental data are available.
Context within the protein-AI landscape
Meta’s ESM family and BioMap’s 100 B-parameter xTrimo model have advanced the field dramatically, yet their training budgets remain prohibitive for most labs. Ainnocence’s leaner AINN-P1 delivers comparable accuracy without billion-scale parameters or MSAs, validating a sequence-only paradigm for broad adoption.
“Sequence is the new paradigm,” said
Dr. Lurong Pan, CEO
. “By learning the language of proteins, we predict complex properties without relying on structures or wet-lab screens. These results rival Big Tech’s best while remaining cost-efficient.”
Integration & impact on
Ainnocence’s platform
The upgraded model is now being rolled into SentinusAI
®
(antibody engineering), CarbonAI
®
(small-molecule & PROTAC design) and CellulaAI
®
(cell-therapy optimization). Higher-fidelity predictions of stability and expression accelerate antibody lead-opt studies, while enhanced activity/binding scores improve small-molecule hit selection – compressing discovery timelines from years to weeks.
Ainnocence invites research groups and biopharma partners to leverage its sequence-first AI platform for protein engineering, vaccine work and hard-to-drug targets. Contact service@ainnocence.com or visit
www.ainnocence.com
for collaboration details.
About Ainnocence
Founded in 2021, Ainnocence is a next-generation biotech company whose self-evolving AI platform can virtually screen 10¹⁰ protein sequences or small-molecule candidates for multitarget and multi-objective optimization, optimizing multiple properties simultaneously to deliver high-probability leads with unprecedented speed and cost efficiency.
Lurong Pan, PhD
Ainnocence
+1 205-249-7424
service@ainnocence.com
Visit us on social media:
LinkedIn
YouTube
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability
for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this
article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
蛋白降解靶向嵌合体
2025-08-13
·生物谷
2025年8月12日,追光生物科技 (深圳)有限公司(以下简称:追光生物)与华大智造宣布达成战略合作关系。此次合作将充分发挥双方在生命科学领域的技术及平台优势,共同打造覆盖“筛选-测序-数据分析”全流程的自主可控的抗体发现平台,加速生命科学仪器的产业化进程。
01
携手共建
自主可控的抗体发现平台
追光生物是一家致力于变革新药发现的创新生命科学公司,从事前沿生命科学仪器以加速生物学研究与开发,其拥有自主知识产权的单细胞功能筛选平台,可从数万细胞中精准识别和分离出最具治疗潜力的“种子”抗体细胞。
华大智造作为生命科技核心工具的提供方,专注于围绕生命中心法则“读、写、存”的核心工具底层技术研发及产品创新,持续为用户提供覆盖生命科学研究及应用中全场景、全生命周期的系统解决方案。其不仅实现了对“激发光”“自发光”“不发光”三种技术路径基因测序产品的全方位战略布局,更是全球首个同时拥有大规模商业量产级短读长与长读长测序产品的企业。
聚焦国内市场前沿需求,双方将紧密合作,进一步推动抗体药物研发的核心瓶颈突破,大大缩短抗体发现周期,为攻克影响国民健康的重大疾病提供源头创新动力。
追光生物首席执行官谢海南博士(左)与华大智造执行副总裁刘健(右)分别代表双方签署合作协议
追光生物首席执行官谢海南博士表示:“我们非常自豪能与中国测序领军企业的华大智造携手。这次合作不仅是商业层面的携手,更是中国本土创新力量协同发展的有力证明,完美契合了国家构建强大、自主的健康产业的战略方针。此次携手充分彰显了我们在中国本土创造世界一流技术的决心与实力。”
华大智造执行副总裁刘健表示:“华大智造作为‘生命科技核心工具缔造者’,致力于为全球用户提供先进设备平台及开放生态体系。我们期待与追光生物携手,共同探索立足本土、惠及全球的药物发现新生态。”
02
三方合力
构筑AI驱动的创新药研发闭环
值得一提的是,此次追光生物与华大智造的合作将进一步与AI制药企业百图生科协同融合。通过百图生科构建的生命科学基础大模型,将对追光生物OptoBot®系列产品平台上产出的海量单细胞功能性数据与对应测序融合数据进行深度解析,从而预测、设计并优化更具临床前景的抗体药物,形成由中国企业主导的“筛选—测序—AI智能驱动”的新一代药物研发闭环体系。
OptoBot®系列产品
追光生物、华大智造及百图生科三方协同构建的全链路平台,展现了药物发现能力从关键技术到系统集成的全面突破,将助力国内药物研发体系走向全面自主、智能驱动的新发展阶段。
关于追光生物 (OptoSeeker Biotech)
追光生物是一家致力于变革新药发现的中国创新生命科学公司,致力于开发前沿生命科学仪器以加速生物学研究与开发。
我们拥有自主知识产权的平台,深度融合了光电镊技术、微流控技术、生物芯片、荧光染色和蛋白质标记等核心技术,提供超高通量的功能性单细胞筛选,快速识别用于抗体和细胞疗法开发的高价值细胞。我们致力于增强中国的生物医药研发实力,为加速创新药物的诞生贡献中国力量。
关于华大智造 (MGI)
深圳华大智造科技股份有限公司(简称华大智造)秉承“创新智造引领生命科技”的理念,专注于围绕生命中心法则“读、写、存”的核心工具底层技术研发及产品创新,持续为用户提供覆盖生命科学研究及应用中全场景、全生命周期的系统解决方案,致力于以基因测序技术为核心的多组学技术推动科研方向的突破以及临床应用转化,协同生命科学与生物技术行业上下游构建开放、合作、共赢的产业生态。
华大智造成立于2016年,截至2024年12月31日,业务布局遍布六大洲110多个国家和地区,在全球已建立9大研发中心、7 大生产基地、9大国际仓库,以及13个客户体验中心,服务累计超过3,300个用户。作为生命科技核心工具的提供方,华大智造不仅实现了对“激发光”“自发光”“不发光”三种技术路径基因测序产品的全方位战略布局,更是全球首个同时拥有大规模商业量产级短读长与长读长测序产品的企业。
关于百图生科 (BioMap)
BioMap百图生科是生命科学基础大模型的全球先行者,其2100亿参数的生物语言大模型,解码基因组、蛋白质、细胞、生物系统的底层规律,作为“生物模拟器”,大幅减少对传统实验模型的依赖,为研发赋能。
目前,BioMap已在药物研发、生物制造、基础科研、医疗健康领域的200余个任务上实现了State-of-the-Art(SOTA)表现,在数十个专业细分领域为700余家全球客户提供大模型驱动的专业智能体(AI Agent),整合数据、模型、实验能力,为用户带来前沿药物发现、生物反应效率提升、生物数据解读和知识获取等应用成果。
高管变更
2025-08-13
2025年8月12日,追光生物科技 (深圳)有限公司(以下简称:追光生物)与华大智造宣布达成战略合作关系。此次合作将充分发挥双方在生命科学领域的技术及平台优势,重点依托华大智造G99基因测序仪、SP-100RS自动化样本制备系统,共同打造覆盖“筛选-测序-数据分析”全流程的自主可控的#抗体 发现平台,加速生命科学仪器的产业化进程。
携手共建
自主可控的抗体发现平台
#追光生物 是一家致力于变革新药发现的创新生命科学公司,从事前沿生命科学仪器以加速生物学研究与开发,其拥有自主知识产权的单细胞功能筛选平台,可从数万细胞中精准识别和分离出最具治疗潜力的“种子”抗体细胞。
#华大智造 作为生命科技核心工具的提供方,专注于围绕生命中心法则“读、写、存”的核心工具底层技术研发及产品创新,持续为用户提供覆盖生命科学研究及应用中全场景、全生命周期的系统解决方案,不仅实现了对“激发光”“自发光”“不发光”三种技术路径基因测序产品的全方位战略布局,更是全球首个同时拥有大规模商业量产级短读长与长读长测序产品的企业。
聚焦国内市场前沿需求,双方将紧密合作,进一步推动抗体#药物研发 的核心瓶颈突破,大大缩短抗体发现周期,为攻克影响国民健康的重大疾病提供源头创新动力。
追光生物首席执行官谢海南博士(左)与华大智造执行副总裁刘健(右)分别代表双方签署合作协议
追光生物首席执行官谢海南博士表示:“我们非常自豪能与中国测序领军企业的华大智造携手。这次合作不仅是商业层面的携手,更是中国本土创新力量协同发展的有力证明,完美契合了国家构建强大、自主的健康产业的战略方针。此次携手充分彰显了我们在中国本土创造世界一流技术的决心与实力。”
华大智造执行副总裁刘健表示:“华大智造作为‘生命科技核心工具缔造者’,致力于为全球用户提供先进设备平台及开放生态体系。我们期待与追光生物携手,共同探索立足本土、惠及全球的药物发现新生态。”
三方合力
构筑AI驱动的创新药研发闭环
值得一提的是,此次追光生物与华大智造的合作将引入#AI 制药企业百图生科的技术支持。通过百图生科构建的生命科学基础大模型,将对追光生物OptoBot®系列产品平台上产出的海量单细胞功能性数据与对应测序融合数据进行深度解析,从而预测、设计并优化更具临床前景的抗体药物,形成由中国企业主导的“筛选—测序—AI智能驱动”的新一代药物研发闭环体系。
追光生物、华大智造及百图生科三方协同构建的全链路平台,展现了药物发现能力从关键技术到系统集成的全面突破,将助力国内药物研发体系走向全面自主、智能驱动的新发展阶段。
截至2024年12月31日,华大智造业务遍及六大洲超过110多个国家和地区,服务累计超过3,300个用户。未来,华大智造将将始终秉承“创新智造引领生命科技”的理念,持续以全套生命数字化设备和系统满足不同应用场景下客户的多样化需求,提供全方位、更高效、更低成本的强大工具,积极拓宽生命科学产品矩阵,助力全球用户大步拥抱多组学浪潮。
关于追光生物
OptoSeeker Biotech
追光生物是一家致力于变革新药发现的中国创新生命科学公司,致力于开发前沿生命科学仪器以加速生物学研究与开发。
我们拥有自主知识产权的平台,深度融合了光电镊技术、微流控技术、生物芯片、荧光染色和蛋白质标记等核心技术,提供超高通量的功能性单细胞筛选,快速识别用于抗体和细胞疗法开发的高价值细胞。我们致力于增强中国的生物医药研发实力,为加速创新药物的诞生贡献中国力量。
关于百图生科
BioMap
BioMap百图生科是生命科学基础大模型的全球先行者,其2100亿参数的生物语言大模型,解码基因组、蛋白质、细胞、生物系统的底层规律,作为“生物模拟器”,大幅减少对传统实验模型的依赖,为研发赋能。
目前,BioMap已在药物研发、生物制造、基础科研、医疗健康领域的200余个任务上实现了State-of-the-Art(SOTA)表现,在数十个专业细分领域为700余家全球客户提供大模型驱动的专业智能体(AI Agent),整合数据、模型、实验能力,为用户带来前沿药物发现、生物反应效率提升、生物数据解读和知识获取等应用成果。
·
·
高管变更
100 项与 百图生科(北京)智能技术有限公司 相关的药物交易
登录后查看更多信息
100 项与 百图生科(北京)智能技术有限公司 相关的转化医学
登录后查看更多信息
组织架构
使用我们的机构树数据加速您的研究。
登录
或

管线布局
2026年02月08日管线快照
管线布局中药物为当前组织机构及其子机构作为药物机构进行统计,早期临床1期并入临床1期,临床1/2期并入临床2期,临床2/3期并入临床3期
药物发现
7
8
临床前
其他
1
登录后查看更多信息
当前项目
| 药物(靶点) | 适应症 | 全球最高研发状态 |
|---|---|---|
CI-09(Biomap Intelligent) | 自身免疫性疾病 更多 | 临床前 |
CI-05(Biomap Intelligent) | 实体瘤 更多 | 临床前 |
CI-03(Biomap Intelligent) | 实体瘤 更多 | 临床前 |
BP-201(Biomap Intelligent) | 自身免疫性疾病 更多 | 临床前 |
CI-01(Biomap Intelligent) | 实体瘤 更多 | 临床前 |
登录后查看更多信息
药物交易
使用我们的药物交易数据加速您的研究。
登录
或

转化医学
使用我们的转化医学数据加速您的研究。
登录
或

营收
使用 Synapse 探索超过 36 万个组织的财务状况。
登录
或

科研基金(NIH)
访问超过 200 万项资助和基金信息,以提升您的研究之旅。
登录
或

投资
深入了解从初创企业到成熟企业的最新公司投资动态。
登录
或

融资
发掘融资趋势以验证和推进您的投资机会。
登录
或

生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用
