预约演示
更新于:2026-02-27
Lumasiran sodium
鲁玛司兰
更新于:2026-02-27
概要
基本信息
非在研机构- |
最高研发阶段批准上市 |
首次获批日期 欧盟 (2020-11-19), |
最高研发阶段(中国)- |
特殊审评孤儿药 (韩国)、孤儿药 (澳大利亚)、突破性疗法 (美国) |
登录后查看时间轴
结构/序列
使用我们的RNA技术数据为新药研发加速。
登录
或
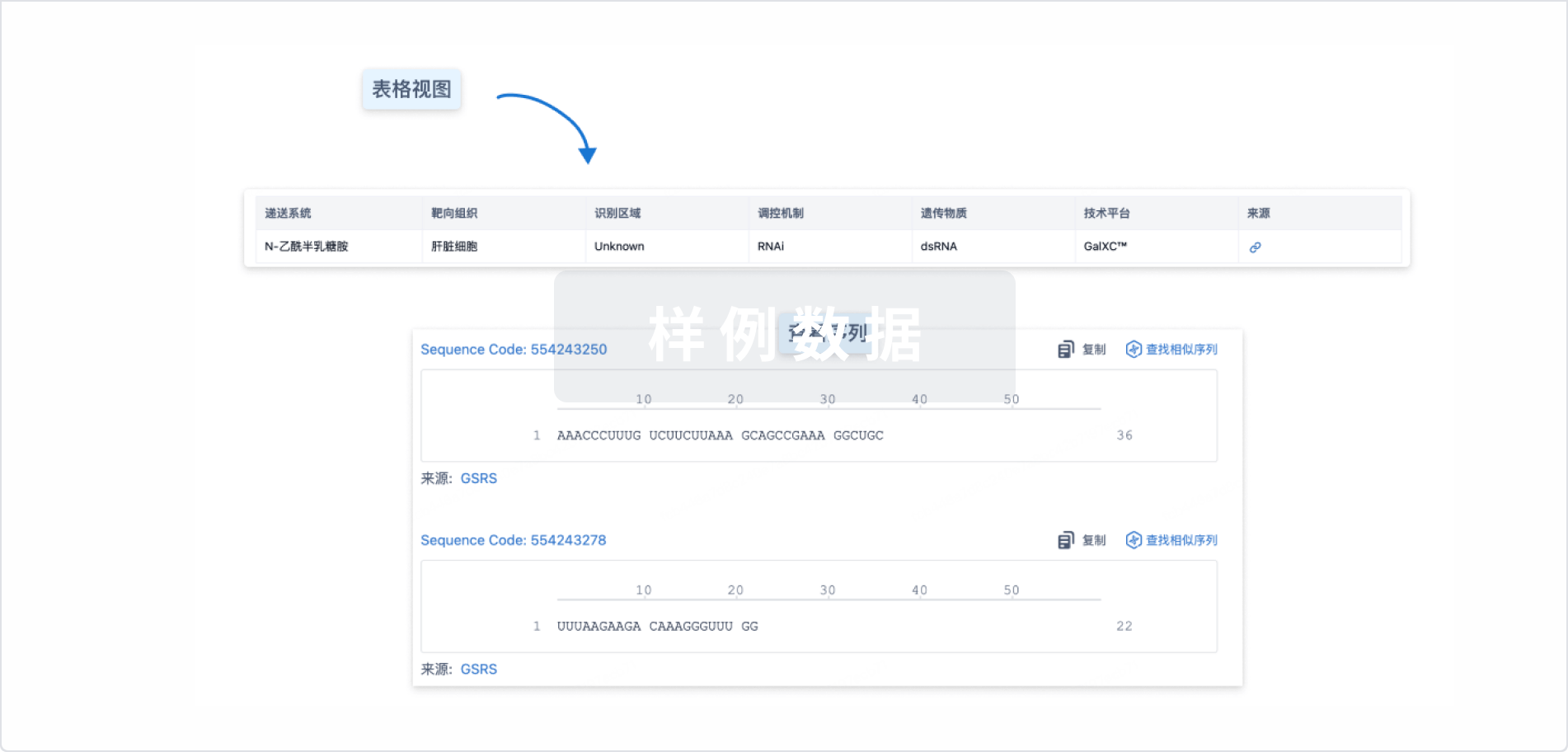
Sequence Code 282408991

来源: *****
Sequence Code 1191632796

来源: *****
关联
9
项与 鲁玛司兰 相关的临床试验NCT06225544
Lumasiran in Hyperoxalaemic Patients on Haemodialysis
This study will look at how well a drug that reduced the amount of oxalate in the body works in patients that have kidney disease and need dialysis treatment. People with kidney disease often have higher levels of oxalate in the blood. People with kidney disease are also at higher risk of having heart attacks, heart disease and strokes (these are called cardiovascular diseases). It is thought that high oxalate levels may increase the risk of these diseases. This study will investigate if this medicine can lower the amount of oxalate in the blood of dialysis patients and see if there is any change in the health of their heart. This medicine is already used for people who have high oxalate levels because of a genetic cause and has been used safely for patients on dialysis.
The study will put the participants randomly into either the group getting the study medicine or the group getting a placebo (this will be a solution of saline water). Neither participants not the doctors will know whether the drug or placebo is given until after the end of the study.
At the start of the study all the participants will have an echocardiogram (an ultrasound of the heart) and again 6 months later at the end of the study. We will also take blood tests once a month when the participants come for dialysis.
The study will put the participants randomly into either the group getting the study medicine or the group getting a placebo (this will be a solution of saline water). Neither participants not the doctors will know whether the drug or placebo is given until after the end of the study.
At the start of the study all the participants will have an echocardiogram (an ultrasound of the heart) and again 6 months later at the end of the study. We will also take blood tests once a month when the participants come for dialysis.
开始日期2024-04-14 |
申办/合作机构 |
NCT06225882
Retrospective and Prospective Follow-up of Patients With Primary Hyperoxaluria Type 1 Treated With Lumasiran in France - DAILY-LUMA
Primary hyperoxaluria type 1 (PH1) is a rare genetic disease caused by mutation in the AGXT gene encoding the hepatic peroxisomal enzyme AGT. Reduced AGT activity results in increased glyoxylate and oxalate production, causing the formation of kidney stones, nephrocalcinosis and renal failure. Clinical trials of Lumasiran have provided information on the efficacy and safety of Lumasiran in the treatment of primary hyperoxaluria type 1. However, they do not provide data on long-term efficacy, safety and patient management. As part of the post-marketing follow-up of Lumasiran, in agreement with the authorities, this study proposes a retrospective and prospective follow-up over 5 years of pediatrics and adults patients treated in France with a standardized clinical, biological and radiological follow-up. The main objective is to monitor the evolution of PH1 parameters and particularly oxaluria before and after treatment.
开始日期2023-01-01 |
申办/合作机构 |
NCT05161936
A Randomized, Double-blind, Placebo-controlled Study to Evaluate the Efficacy, Safety, Pharmacodynamics, and Pharmacokinetics of Lumasiran in Patients With Recurrent Calcium Oxalate Kidney Stone Disease and Elevated Urinary Oxalate Levels
The primary purpose of this study is to evaluate the effect of lumasiran on the percent change in urinary oxalate excretion in patients with recurrent calcium oxalate kidney stone disease.
开始日期2022-01-27 |
100 项与 鲁玛司兰 相关的临床结果
登录后查看更多信息
100 项与 鲁玛司兰 相关的转化医学
登录后查看更多信息
100 项与 鲁玛司兰 相关的专利(医药)
登录后查看更多信息
68
项与 鲁玛司兰 相关的文献(医药)2026-03-01·CLINICA CHIMICA ACTA
The oxalobiome: unraveling the role of gut microbiota in oxalate metabolism and its implications for kidney health and disease management
Review
作者: Wang, Yanzhe ; Minalla, Ahmed ; Gonzales, Karina Ordaya ; Mburu, David ; Namagerdi, Asadoor Amirkhani ; Ali, Bilal ; Bai, Karoona ; Abdelhalim, Khalid A ; Kumar, Sumeet
The oxalobiome, comprising microbial communities involved in oxalate metabolism, plays a critical role in maintaining oxalate homeostasis and preventing associated health issues, particularly calcium oxalate nephrolithiasis. Key organisms, notably Oxalobacter formigenes, are essential for degrading oxalate, yet their abundance is influenced by factors such as diet, genetics, and antibiotic use. Recent advances in research have elucidated the complex interactions between the gut microbiome and oxalate metabolism, highlighting the potential for therapeutic interventions. Innovative strategies, including RNA interference therapies (e.g., lumasiran, nedosiran), engineered probiotics, and gene-editing technologies, show promise in managing conditions like primary hyperoxaluria. However, challenges remain, including limitations in oxalate measurement techniques and variability in microbial populations. Multi-omics approaches and metagenomic analyses have enhanced our understanding of the oxalobiome, revealing novel microbial taxa and metabolic pathways involved in oxalate degradation. Despite the potential of emerging therapies, clinical translation is still in its infancy, necessitating further research to establish efficacy and safety. Future studies should focus on mechanistic insights, standardized methodologies, and targeted microbiome-based therapies to optimize management strategies for hyperoxaluria and related systemic diseases. A comprehensive understanding of the oxalobiome is essential for developing precision medicine approaches that effectively address oxalate dysregulation and improve patient outcomes.
2026-02-01·Nucleic Acid Therapeutics
Class-Specific Adverse Events of Patients Treated with Small Interfering RNA Therapeutics: A Disproportionality Analysis of the United States Food and Drug Administration Adverse Event Reporting System Database Based on the MY FAERS Platform
Article
作者: Sun, Yiqi ; Li, Ze ; Wang, Xiaozhen ; Li, Dandan ; Li, Xingang ; Zhang, Lin
Small interfering RNA (siRNA) therapeutics represent a transformative class of drugs, but their class-specific adverse events (CAE-siRNA) remain incompletely characterized. This study aimed to identify and quantify CAE-siRNA associated with U.S. Food and Drug Administration (FDA)-approved siRNA drugs (patisiran, givosiran, vutrisiran, inclisiran, and lumasiran) using real-world pharmacovigilance data, focusing on potential class-wide effects. A disproportionality analysis was conducted using the FDA Adverse Event Reporting System database (2014–2025Q2) accessed via the MY FAERS platform. The reporting odds ratio (ROR) with 95% confidence interval (CI) was calculated, with signals defined by a lower CI >1 and ≥3 cases. Sensitivity analyses included indication-matched populations (IMPs) and exclusion of concomitant medications. Causality was assessed using Bradford Hill criteria. Among 6200 siRNA-treated patients, 45 CAE-siRNA spanning 10 system organ classes were identified. Pain and pain in extremity, fatigue, and gastrointestinal disorders were the most frequently reported. Notably, patisiran was associated with an elevated risk of back pain (ROR: 2.28, 95% CI: 1.84–2.83), whereas givosiran exhibited significant signals for stress (ROR: 5.29, 95% CI: 3.64–7.70) and weight loss (ROR: 2.35, 95% CI: 1.74–3.16). Of particular concern, inclisiran demonstrated strong hepatic toxicity signals (ROR ranging from 9.11 to 86.06) along with discomfort (ROR: 3.60, 95% CI: 1.34–9.65). Sensitivity analyses confirmed robustness across subgroups. Furthermore, causality assessment supported a likely association between the hepatic toxicity and inclisiran. This study identified clinically relevant CAE-siRNA, particularly hepatic toxicity for inclisiran, supporting enhanced monitoring. While disproportionality analyses are hypothesis generating, these findings underscore the need for targeted pharmacovigilance to optimize the safety of this promising drug class.
2026-02-01·CLINICAL TRANSPLANTATION
A Case of Successful Kidney Transplant‐Alone in Primary Hyperoxaluria Type 1 Using Lumasiran
Article
作者: Alhamad, Tarek ; Abuazzam, Farah ; Flores, Karen ; Abdulkhalek, Samer ; Java, Anuja ; Merzkani, Massini ; Gaut, Joseph ; Paul, Rohan ; Delos Santos, Rowena
ABSTRACT:
Simultaneous liver‐kidney transplantation (SLKT) has been the standard of care for patients with end‐stage renal disease (ESRD) and primary hyperoxaluria type 1 (PH1). Lumasiran is a novel RNA interference (RNAi) therapy that targets glycolate oxidase and decreases oxalate production in the liver. Despite its availability, the use of RNAi with kidney transplant alone (KTA) for ESRD related to primary hyperoxaluria remains limited. We report a 39‐year‐old female with a history of KTA who presented with diarrhea and severe AKI. She rapidly progressed to anuria and allograft loss. Her kidney biopsy demonstrated calcium oxalate deposition. The patient was subsequently evaluated for a repeat kidney transplant. Given the morbidity associated with SLKT, RNAi therapy was pursued as an alternative. Initiation of Lumasiran reduced serum oxalate from 40 to 8 µmol/L, enabling successful KTA with immediate graft function. At 12 months post‐transplant, renal function remained stable (creatinine 0.82 mg/dL, serum oxalate <1.5 µmol/L). This case highlights RNAi therapy as a promising strategy to facilitate KTA in patients with PH1 and ESRD, potentially reducing the need for SLKT and its associated morbidity.
295
项与 鲁玛司兰 相关的新闻(医药)2026-02-17
·生命科学
RNA疗法技术创新与治疗应用年度发展态势
杨若南,施慧琳,王 琼,张永娟,孙迟临,李祯祺*
(中国科学院上海生命科学信息中心,中国科学院上海营养与健康研究所,上海200031)
摘 要:随着化学修饰技术、递送技术的协同创新突破,以小核酸药物与mRNA药物为代表的RNA疗法领域正快速迈向临床转化与应用拓展的新阶段,并迅速崛起为全球生物医药创新的核心前沿。本文系统梳理分析了2025年RNA疗法在技术、基础和临床研究及产业化方面的研究进展,研判未来发展趋势,并提出未来重点方向。研究发现,序列设计、化学修饰技术与递送系统不断优化演进,向精细化、智能化与多器官递送发展,人工智能赋能显著提升研发效率与精准度。同时,对RNA疗法作用机制、治疗策略及新兴技术潜力的不断深化与探索,共同推动RNA疗法在临床应用上从遗传病、传染病等向癌症、心血管疾病等慢性病延伸,产业规模进一步扩大,而中国正成为关键技术发源地。
关键词:RNA疗法;小核酸药物;mRNA疫苗/药物;递送技术
RNA疗法主要是指以RNA为活性成分,通过调控基因表达或补充功能性蛋白等发挥治疗作用的疗法,其理论突破催生了RNA药物的诞生。依据分子大小的不同,RNA药物目前主要可分为小核酸药物和大分子RNA药物,小核酸药物通常由15-30个核苷酸组成,包括单链或双链结构。从作用机制来看,小核酸药物可细分为作用于基因调控层面的反义寡核苷酸(antisense oligonucleotides,ASO)、小干扰RNA(small interfering RNA,siRNA)、微小RNA(microRNA,miRNA)、小激活RNA(small activating RNA,saRNA)等,以及直接靶向蛋白质的适配体(aptamer)。小核酸药物通过碱基互补配对结合靶标RNA或DNA,通过不同机制精准调控基因表达。与小分子、抗体等传统药物相比,此类药物具有高度特异性,可通过序列设计靶向传统药物难以触及的致病基因,在mRNA层面实现精准调控,目前已广泛应用于遗传病、肿瘤、病毒感染及代谢性疾病等领域。大分子RNA药物目前主要包括信使RNA(messenger RNA,mRNA)和环状RNA(circular RNA,circRNA)。mRNA通过在细胞内被核糖体翻译,直接指导合成治疗性蛋白或抗原,用于疫苗开发具有快速设计与生产、灵活快速应对病毒变异等优势。新兴的环状RNA因其独特的闭合环状结构,稳定性与持续表达潜力较线性RNA更优。经工程改造的circRNA不仅可实现蛋白质翻译,还可作为miRNA海绵、蛋白质支架或免疫传感器配体,参与基因调控、信号转导和免疫激活,展现出作为疫苗与治疗药物的多重应用前景(图1)。
图1 RNA疗法研发体系图
早期RNA分子因体内稳定性差、递送效率低和天然免疫原性较强等核心挑战而发展缓慢。近年来,随着基础研究的不断深入、关键技术的不断突破、临床转化与产业化进程的持续加速,该领域步入产业化爆发阶段。截至2025年,全球已有23款小核酸药物获批,其中ASO药物13款、siRNA药物8款、RNA适配体药物2款。此外,还有多款用于新冠病毒及呼吸道合胞病毒(respiratory syncytial virus,RSV)感染的mRNA疫苗获批上市。其中,2025年全球获批上市的siRNA药物2款,ASO药物1款。1 RNA疗法的技术创新进展
RNA药物在开发过程中面临一系列固有挑战:其在体内容易被核酸酶快速降解,因分子本身携带负电荷而难以跨越细胞膜屏障,且易引发不必要的先天免疫反应。为系统克服这些瓶颈,需对RNA序列与结构进行理性设计,从源头优化其功能特性;进一步,进行核苷酸的骨架、核糖或碱基的化学修饰,以显著增强其稳定性、降低免疫原性并改善其药代动力学行为;最后构建高效低毒的递送系统,以保护RNA分子、协助其进入靶细胞,并实现组织特异性递送。1.1 序列结构设计精细化,AI驱动理性设计突破
序列结构设计是RNA药物发挥疗效的关键,针对不同类型RNA药物的作用机制,其设计策略也存在显著差异。小核酸药物的序列设计是其靶向作用的基础,核心在于通过精确的碱基配对实现对目标RNA的高特异性和高亲和力识别,从而决定治疗活性与脱靶风险。而mRNA药物的序列设计主要通过优化编码区和非翻译区来调控蛋白质表达水平、分子半衰期及免疫原性。例如,针对疫苗应用,可保留适度免疫刺激性以增强免疫应答;用于蛋白质替代治疗时,则需实现高效、持久且安全的蛋白质表达,已有研究证实优化mRNA非翻译区元件能显著提升蛋白产量[1]。同时,由于序列较长的mRNA通常免疫原性较高,需最大限度降低免疫激活。在此基础上,新兴的环状mRNA凭借闭合环状结构展现出优于线性mRNA的稳定性和更低的免疫原性。然而,传统环状mRNA缺乏5′端帽子结构,主要依赖内部核糖体进入位点(IRES)启动翻译,效率受限。针对这一瓶颈,日本名古屋大学的研究人员通过共价或非共价两种方式引入m7G帽结构,使环状mRNA的翻译效率较使用IRES序列提升了200倍,且该技术与化学修饰兼容,进一步减少了免疫刺激效应[2]。
随着对核酸结构与功能关系认知的深入,序列设计已从早期的经验依赖和规则驱动,进入人工智能(AI)驱动的理性设计阶段,有望解决传统设计过程繁琐、效率低下的问题,生成式模型在mRNA优化与ASO靶点筛选中成效初显。北京衡昱生物与美国麻省理工学院联合开发的生成式AI驱动平台GEMORNA,可进行mRNA序列的定向设计与优化,有效兼顾其翻译效率与稳定性[3]。原中国科学院上海免疫与感染研究所(现上海药物研究所)也推出了AI辅助设计平台mRNAdesigner,降低了设计门槛[4]。针对小核酸药物,eSkipFinder[5]和ASOptimizer[6]分别解决了外显子跳跃ASO设计和高效靶点筛选的需求。然而,现有AI工具在跨靶点泛化能力、协同优化方面仍显不足,且对非编码区调控元件的系统性设计规则尚未建立。未来构建可解释、可迁移的智能设计平台,是技术突破的重点方向。1.2 化学修饰技术持续优化,从稳定性提升到智能化调控
化学修饰对优化ASO、siRNA、mRNA和适配体的稳定性、特异性、安全性和疗效至关重要。目前的修饰类型主要包括:①磷酸骨架:硫代磷酸酯(PS)和磷酸二氨基吗啉代寡核苷酸(PMO)等;②核糖:2′-O-甲基(2′-OMe)、2′-氟(2′-F)、2′-甲氧基乙基(2′-MOE)等;③碱基:假尿苷(Ψ)、N1-甲基假尿苷(m¹Ψ)及5-甲基胞嘧啶等;④末端:5′端加帽和3′端修饰;⑤偶联化合物:N-乙酰半乳糖胺(GalNAc)、抗体、脂质、肽等。不同RNA药物需依据作用机制和序列组成进行独特修饰。
目前的小核酸药物的化学修饰主要集中在2′‑OMe、2′‑MOE、2′‑F等2′‑糖环修饰和硫代磷酸骨架,以及对特定分子的共价共轭。此外,具有独特构象调控作用的修饰,如锁核酸与解锁核酸为药物设计提供了更精细的调控手段。近年来,通过化学修饰技术实现小核酸药物的靶向智能化与响应动态化成为新的方向之一。利用pH、酶或光等环境敏感连接子构建前药型寡核苷酸,可实现病理微环境选择性激活,显著降低了脱靶效应,提高了药物的可控性和安全性。例如,德国明斯特大学等机构开发了一种基于光控的磷二胺吗啉寡核苷酸嵌合体,在活体斑马鱼胚胎中实现了对目标蛋白表达的精确光诱导[7]。北京大学开发了一种酶响应型环状ASO,仅在肿瘤细胞高表达的NQO1酶激活下释放线性ASO,促进靶标RNA降解[8]。然而,这种微环境响应型动态调控仍面临体内复杂微环境中响应元件的激活精准度不足,以及前药释放动力学与治疗窗的匹配难题等核心挑战。
mRNA、circRNA等可翻译RNA的化学修饰旨在精准平衡稳定性、翻译效率与免疫原性。目前,碱基修饰是其提升成药性的主流策略,尤以Ψ及其衍生物m¹Ψ最为关键,它们已成功应用于上市mRNA疫苗。这些修饰能有效逃避天然免疫识别并大幅提升翻译效率,近期研究进一步揭示了其免疫逃逸机制,如有研究揭示Ψ修饰能改变RNA构象使其免于被溶酶体核酸酶切割,且避免被TLR7/8免疫受体识别,从而实现免疫规避[9];另有研究发现E3泛素连接酶TRIM25可通过质子感知,特异性地识别并清除通过内吞途径进入细胞的外源RNA,并解释了常用的m1Ψ修饰通过降低mRNA与TRIM25的结合,助其逃逸细胞质层的免疫监视[10]。此外,m⁵C等修饰亦能通过调节密码子-反密码子相互作用和核糖体动力学提高蛋白产量。除碱基修饰外,核糖2′位修饰虽在小核酸药物中广泛应用于增强核酸酶稳定性,但受限于体外转录(IVT)工艺,长链mRNA难以实现该类位点特异性修饰。近期,日本京都大学等机构通过完全化学合成方法,成功在mRNA开放阅读框密码子首位引入位点特异性2'-F修饰,在显著增强稳定性的同时维持了翻译效率,为解决这一难题提供了新路径[11]。但该技术路径面临合成成本高、对蛋白质折叠及长期安全性影响不明等瓶颈。1.3 递送技术多元化发展,肝外靶向持续突破
高效、靶向的递送技术是RNA药物发展的核心。目前最为成熟的递送技术主要为脂质纳米粒(lipid nanoparticle,LNP)和GalNAc偶联技术。其中,LNP由可电离脂质、辅助磷脂、胆固醇及聚乙二醇修饰的脂质组成,凭借其高效封装能力、核酸酶防护及内体逃逸机制,已成为mRNA疫苗的主流载体,早期曾获批应用于siRNA药物。GalNAc则通过共价连接三价配体,靶向肝细胞高表达的去唾液酸糖蛋白受体,实现寡核苷酸的高效肝富集,推动了多款siRNA药物的成功上市。
然而,现有技术仍存在显著局限。LNP系统面临递送效率与生物安全性难以兼顾、易引发固有免疫反应等核心挑战。更为重要的是,由于LNP的天然肝趋向性和GalNAc技术的肝组织特异性,向中枢神经系统、实体肿瘤、肺部等复杂肝外器官实现系统性精准递送仍是领域内的根本性挑战,极大地限制了RNA药物的临床应用范围。因此,RNA药物递送技术目前主要聚焦于肝靶向LNP载体性能的深度优化与肝外组织的主动靶向突破。
为提升LNP的整体性能,研究人员正从理论、组分及筛选手段等多维度对其进行迭代升级。近期研究发现,LNP不仅能递送药物,还能辅助抗原递呈并调节细胞定位,与mRNA药物共同作用优化T细胞免疫应答[12]。在组分优化方面,威斯津生物利用多氮可电离脂质替代传统单氮脂质,有效解决了内涵体逃逸效率与脂质毒性难以平衡的难题,基于该LNP的多款mRNA药物已进入临床阶段;中国科学技术大学则通过系统调控N/P比与组分配比,成功构建了低免疫原性LNP递送平台,显著降低了由载体引起的非特异性炎症反应[13]。美国麻省理工学院研发的新型纳米颗粒AMG1541采用具有环状结构与酯键尾部的可电离脂质,通过显著提升内体释放效率,实现剂量降低至原来的1/100,有效平衡了高递送效率与生物可降解性[14]。在筛选手段上,AI技术的应用极大解决了多组分系统的组合优化难题。剂泰科技的AI纳米递送平台NanoForge可通过高效搜索与虚拟筛选,将LNP研发周期从数月缩短至数天。美国麻省理工学院开发的深度学习模型COMET,能建模LNP组分的复杂相互作用并精准预测递送效率,成功筛选出高性能新配方[15]。
为实现多组织器官的特异性靶向,研究人员正积极开拓更为多元化、具备主动靶向能力的新型递送工具箱。一种策略是改造LNP。美国西奈山伊坎医学院在改造LNP实现大脑与肺等器官靶向方面取得系列重要进展:如通过将可跨越血脑屏障的小分子结构整合入LNP核心脂质,开发了MK16 BLNP系统,可将mRNA高效递送至大脑神经元与星形胶质细胞[16];利用血清素配体SR-57227衍生的可电离脂质,并结合细胞穿透肽Tat进行表面修饰开发了OS4T LNP,可实现胶质母细胞瘤的特异性靶向,白介素-12 mRNA的递送与表达效率提升50倍以上[17];将编码特定抗菌肽的mRNA以具备抗炎功能的三硫键脂质纳米颗粒包裹,有效递送至肺部,克服了传统抗菌肽递送难和半衰期短的问题[18]。除对LNP改造外,GalNAc技术在肝靶向上的成功,为配体/抗体介导的主动靶向等肝外递送策略提供了重要的范式参考,但肝外递送需根据不同组织的受体和微环境,重新设计与筛选适配的配体体系。抗体-寡核苷酸偶联物(antibody–oligonucleotide conjugate,AOC)技术可通过抗体靶向特定受体实现向肌肉、心肌及中枢神经系统等肝外组织的主动靶向。美国耶鲁大学利用单抗TMAB3将治疗性RNA递送至高表达转运蛋白ENT2的癌细胞中,递送效率较正常细胞提高1 500倍,有效抑制了肿瘤生长[19]。诺华旗下的美国Avidity Biosciences(以下简称Avidity)和Dyne Therapeutics等公司在AOC领域处于领先地位,采用靶向转铁蛋白受体1(transferrin receptor 1,TfR1)的单抗或抗原结合片段实现siRNA向肌肉组织的靶向递送,其中Avidity公司开发的Del-desiran作为首个基于TfR1的肌肉递送siRNA产品已进入Ⅲ期临床。美国Alnylam公司开发的2'-O-十六烷基(C16)共轭技术可实现对中枢神经系统、眼部和肺部等非肝脏组织的靶向递送。基于该技术开发用于阿尔茨海默病的siRNA药物ALN-APP,目前已取得初步的临床验证。尽管AOC、C16共轭等技术为肝外递送开辟了新路径,但其疗效仍受限于靶向配体的体内稳定性与非肝组织的内体逃逸效率这两大瓶颈。未来需要在提升靶向穿透能力的同时,全面评估新型递送系统的长期安全性。2 RNA疗法的基础与临床研究进展
2025年,RNA疗法领域迎来了丰收之年,多款产品的获批以及多项关键临床试验结果的公布,验证了各项技术平台尤其是小核酸药物和mRNA技术的可行性与潜力。而对其作用机制的深入理解、治疗策略与应用的创新探索将进一步推动药物和疫苗的临床转化进程。环状RNA疗法作为新兴技术目前已快速步入临床概念验证阶段。2.1 小核酸药物
目前全球已获批的小核酸药物主要集中于罕见遗传性疾病(表1),以ASO和siRNA药物为主。从作用机制来看,siRNA药物和部分ASO药物通过诱导靶mRNA降解实现基因沉默,而另一部分ASO药物则通过纠正RNA剪接来恢复基因功能。
表1全球已获批的小核酸药物
名称
研发机构
适应证
批准时间/机构
给药方式
类型
福米韦生(Fomivirsen)
美国Ionis公司、瑞士诺华公司
CMV视网膜炎
1998年FDA
玻璃体
ASO
Macugen
美国辉瑞公司、Eyetech
新生血管性年龄相关性黄斑变性
2004年
玻璃体
适配体
米泊美生(Mipomersen)
美国Ionis公司、法国赛诺菲
纯合子型家族性高胆固醇血症
2013年FDA
皮下
ASO
Eteplirsen
美国Sarepta公司
51外显子跳跃杜氏肌营养不良症
2016年FDA
静脉
ASO
诺西那生(Nusinersen)
美国Ionis公司、美国渤健制药公司
脊髓性肌萎缩症
2016年FDA
鞘内
ASO
Inotersen
美国Ionis公司
遗传性转甲状腺素介导的淀粉样变性多发性神经病
2018年FDA
静脉
ASO
Patisiran
美国Alnylam公司
遗传性转甲状腺素介导的淀粉样变性多发性神经病
2018年FDA
静脉
siRNA
Volanesorsen
美国Ionis公司
家族性乳糜微粒血症综合征
2019年EMA
皮下
ASO
Givosiran
美国Alnylam公司
成人急性肝卟啉症
2019年FDA
皮下
siRNA
Golodirsen
美国Sarepta公司
53外显子跳跃杜氏肌营养不良症
2019年FDA
静脉
ASO
Viltolarsen
日本新药株式会社(Nippon Shinyaku)
53外显子跳跃杜氏肌营养不良症
2020年FDA
静脉
ASO
Lumasiran
美国Alnylam公司
1型原发性高草酸尿症
2020年EMA
皮下
siRNA
Inclisiran
美国Alnylam公司、诺华
纯合子家族性高胆固醇血症
2020年EMA、2021年FDA
皮下
siRNA
Casimersen
美国Sarepta公司
45外显子跳跃杜氏肌营养不良症
2021年FDA
静脉
ASO
Vutrisiran
美国Alnylam公司
遗传性转甲状腺素蛋白介导(hATTR)淀粉样变性
2022年FDA、EMA
皮下
siRNA
Tofersen
美国Biogen公司、美国Ionis公司
超氧化物歧化酶1(SOD1)突变所致的肌萎缩侧索硬化
2023年FDA
鞘内
ASO
Nedosiran
美国Dicerna Pharmaceuticals(诺和诺德收购)
1型原发性高草酸尿症
2023年FDA
皮下
siRNA
Izervay
美国Iveric Bio(安斯泰来收购)
干性黄斑变性继发的地理萎缩(GA)
2023年FDA
玻璃体
适配体
Eplontersen
美国Ionis公司
遗传性转甲状腺素介导的淀粉样变性多发性神经病(ATTRv-PN)
2023年FDA
皮下
ASO
Olezarsen
美国Ionis公司
家族性乳糜微粒血症综合征
2024年FDA
皮下
ASO
Fitusiran
法国赛诺菲、美国Alnylam公司
血友病A/B
2025年FDA
皮下
siRNA
Donidalorsen
美国Ionis公司
遗传性血管性水肿
2025年FDA
皮下
ASO
Plozasiran
法国赛诺菲、美国Arrowhead公司
家族性乳糜微粒血症综合征
2025年FDA
皮下
siRNA
注:表格中FDA为美国食品药品监督管理局,EMA为欧洲药品管理局。
随着基础研究的深入,小核酸药物的应用边界正快速扩展。有研究利用患者来源类器官构建疾病模型以精准筛选候选药物[20],探索产前ASO干预将脊髓性肌萎缩症治疗窗口前移[21],并尝试通过调控PD-L1等免疫检查点重塑肿瘤微环境[22]。这些探索为小核酸药物拓展至更广泛疾病奠定基础。长效降脂siRNA药物克司兰钠(Inclisiran)的全球获批标志小核酸药物进入重大慢性病领域,此后,针对高血压、代谢性脂肪肝、乙肝、阿尔茨海默病乃至肥胖症等高发慢病的小核酸药物研发开始加速推进,其适应证正逐渐实现从遗传病/罕见病向常见慢性病的跨越。2.1.1 小核酸药物的遗传性罕见病治疗临床进展
小核酸药物研发在机制复杂的罕见病领域不断深入和延伸,并形成了从临床早期探索到获批上市再到迭代优化的完整布局,其治疗策略主要围绕基因沉默与RNA剪接修正两大核心机制展开。
基于基因沉默策略,小核酸药物研发在突破新适应证的同时,也在不断实现迭代升级。2025年获批的用于遗传性血管性水肿的Donidalorsen和用于血友病的Fitusiran填补了相关领域的小核酸药物空白。临床在研药物中,多款针对罕见神经系统及肌肉疾病的药物已进入临床Ⅲ期(表2)。其中美国Ionis公司开发的ION582和Ultragenyx公司的Apazunersen,于2025年先后获得美国食品药品监督管理局(Food and Drug Administration,FDA)用于治疗天使综合征的突破性疗法认定。Avidity公司的AOC药物Delpacibart etedesiran有望成为1型强直性肌营养不良首个获批的突破性疗法。美国Ionis公司的Zilganersen在针对亚历山大病的关键研究中取得积极顶线结果,成为首个证明具有临床意义和疾病改善作用的研究药物,已获美国FDA突破性疗法、孤儿药等多项认定。此外,另有多款处于临床Ⅰ期的在研药物也展现出良好潜力。瑞士罗氏公司针对天使综合征的Rugonersen在Ⅰ期临床试验中呈现出良好的安全性及症状改善效果[23]。德国慕尼黑大学等机构利用ASO药物Elsunersen对早发型SCN2A发育性癫痫性脑病患儿的治疗尝试,初步表明其安全性良好并能减少癫痫发作[24]。
对于已有药物获批的疾病,新一代疗法借助技术优化,在给药频率、安全性以及患者依从性方面实现了显著提升。以家族性高乳糜微粒血症为例,此前已有两款ASO药物获批上市,分别为美国Ionis公司于2019年获批的Volanesorsen和2024年获批的Olezarsen,2025年获批的普乐司兰钠(Plozasiran)是首个用于该疾病的siRNA药物。这三款药物均以载脂蛋白C-Ⅲ(apolipoprotein C-Ⅲ,APOC3)为靶点,但给药频率从每周一次降至每月一次再到现在的每三个月一次,患者依从性显著提高。同样地,在转甲状腺素蛋白淀粉样变性治疗领域,新一代在研药物Nucresiran可实现每半年或一年给药一次,相较于此前获批的Patisiran、Vutrisiran、Eplontersen,给药频率也进一步降低。
基于RNA剪接修正机制直接修复基因功能的ASO药物,主要用于脊髓性肌肉萎缩症、杜氏肌营养不良症(Duchenne muscular dystrophy,DMD)等遗传病的治疗。如诺西那生钠(Nusinersen)通过促进SMN2基因第7号外显子的包含,有效提升功能性SMN蛋白水平。而在DMD领域,Eteplirsen、Golodirsen、Viltolarsen和casimersen等药物则通过诱导特定致病外显子跳跃,恢复抗肌萎缩蛋白编码序列的阅读框,产生虽截短但具有部分功能的重组蛋白。2025年,该领域持续取得突破性进展,尤其是针对外显子44跳跃的DMD。其中,Avidity公司的抗体偶联药物Delpacibart zotadirsen(del-zota)最新临床数据显示,持续接受治疗一年的患者在多项运动功能指标上呈现出显著改善,逆转了疾病进展的自然轨迹,该药物已获得美国FDA授予的突破性疗法认定。与此同时,另一款ASO药物Brogidirsen在其Ⅰ/Ⅱ期临床研究中也显示出可显著恢复患者肌肉中的抗肌萎缩蛋白水平[25]。
表2 罕见遗传性疾病领域Ⅲ期临床及计划申请上市阶段的小核酸药物
药物类型
药品名称
研发机构
靶点
适应证
递送技术
ASO
Apazunersen
美国Ultragenyx Pharmaceutical公司
UBE3A-ATS
天使综合征
-
Ion582
美国Ionis公司
UBE3A-ATS
天使综合征
-
Elsunersen
美国Ionis公司
Nav1.2
发育性癫痫性脑病
-
Salanersen
美国渤健公司、美国Ionis公司
SMN
脊髓性肌萎缩症
-
Sepofarsen
法国Théa公司、荷兰ProQR公司
CEP290
先天性黑蒙症、Leber遗传性视神经病变
-
Ulefnersen
日本大冢制药、美国Ionis公司
FUS
肌萎缩侧索硬化症
-
Zilganersen
美国Ionis公司
GFAP
亚历山大病
-
Zorevunersen
美国渤健公司、美国Stoke Therapeutics公司
Nav1.1
癫痫发作、Dravet综合征
-
Ultevursen
法国Théa公司、荷兰ProQR公司
usherin
视网膜色素变性、ⅡA型乌谢尔综合征
-
siRNA
ADX-324
美国Adarx公司
KLKB1
遗传性血管性水肿
GalNac
Cemdisiran
美国Alnylam公司、美国再生元公司
C5
重症肌无力
GalNac
Fazirsiran
日本武田、美国Arrowhead公司
A1AT
α1-抗胰蛋白酶缺乏症
GalNac
Nucresiran
美国Alnylam公司
TTR
转甲状腺素蛋白淀粉样变/转甲状腺素蛋白淀粉样变性心肌病、转甲状腺素蛋白家族性淀粉样多发性神经病
GalNac
Zodasiran
美国Arrowhead公司
ANGPTL3
纯合子型家族性高胆固醇血症
GalNac
Delpacibart etedesiran
美国Avidity Biosciences公司(瑞士诺华旗下)
DMPK
强直性肌营养不良
AOC
Delpacibart braxlosiran
美国Avidity Biosciences公司(瑞士诺华旗下)
DUX4
面肩肱型肌营养不良症
AOC
Delpacibart Zotadirsen
美国Avidity Biosciences公司(瑞士诺华旗下)
DMD基因外显子44
杜氏肌营养不良症
AOC
2.1.2 小核酸药物的慢病治疗临床进展
除罕见疾病外,基于基因沉默这一核心机制,小核酸药物正从罕见病延伸至心血管代谢疾病、感染性疾病、神经退行性疾病及肥胖症等主流慢性病领域,彰显出变革未来慢性疾病管理模式的巨大潜能。
心血管代谢疾病是当前小核酸药物最活跃的研发方向之一。继靶向PCSK9的已上市siRNA药物Inclisiran验证了半年一次给药的长效降脂可行性后,针对其他关键血脂靶点的研究快速推进。例如针对新兴风险标志物脂蛋白a[Lp(a)],安进的Olpasiran[26]、礼来的Lepodisiran[27]以及诺华的Pelacarsen等多款药物已进入临床后期阶段。同时,针对甘油三酯代谢,礼来的Solbinsiran[28]通过靶向肝脏ANGPTL3 mRNA,为混合型血脂异常患者提供了新的治疗选择,目前正处于Ⅱ期临床。这些作用于不同通路的药物为血脂异常的个性化综合管理提供了更多可能。在高血压治疗方面,以靶向血管紧张素原(angiotensinogen,AGT)的siRNA为代表的新一代疗法正取得突破。例如,美国Alnylam与罗氏开发的Zilebesiran在Ⅱ期临床试验中显示,单次皮下注射可长效降低血清AGT水平与收缩压达数月之久[29],并已基于积极数据推进至关键Ⅲ期研究,有望将高血压治疗从每日服药革新为数月一次干预。此外,针对代谢功能障碍相关脂肪性肝炎,靶向二酰基甘油酰基转移酶2(diacylglycerol acyltransferase 2,DGAT2)的ASO药物ION224在Ⅱ期临床试验中显示可显著改善患者肝脏组织学特征[30]。
在慢性感染性疾病方面,乙肝领域的小核酸药物研发进展迅速。美国Ionis公司与葛兰素史克(GSK)开发的ASO药物Deprovision的Ⅲ期临床试验正稳步推进,计划于2026年提交上市申请。我国浩博医药的ASO药物AHB-137也已进入Ⅲ期临床,近期公布的Ⅱa期临床试验数据显示,其24周单药治疗实现了30%的临床治愈率。
小核酸药物也为阿尔茨海默病等神经退行性疾病带来治疗新希望。目前临床在研药物采取了差异化的靶点策略。如美国Alnylam公司的Mivelsiran(ALN-APP)与美国Ionis公司的ION269靶向淀粉样前体蛋白(APP)基因,而美国Arrowhead公司的ARO-MAPT及美国Ionis公司的BIIB080则靶向微管相关蛋白tau基因。
针对减重这一热门新兴领域,国内外多家公司正积极布局siRNA药物,靶点上主要围绕INHBE和脂肪细胞的ALK7。美国Wave公司的WVE-007和美国Arrowhead公司的ARO-INHBE、ARO-ALK7,均在早期临床试验中已率先显示出积极的减脂潜力,单次治疗或与GLP-1类药物联用可使内脏脂肪、肝脏脂肪等多个关键指标显著减少。其中,得益于美国Arrowhead公司的肝外递送技术的突破,ARO-ALK7成为全球首款靶向脂肪细胞ALK7并取得初步临床验证的siRNA药物。2.2 mRNA疫苗/药物
以新冠mRNA疫苗的成功为起点,mRNA技术已从应对突发公共卫生事件的应急工具,快速演变为一个高度可编程、模块化的体内蛋白质合成平台。通过序列设计即可编码任意蛋白,实现抗原、酶、抗体及细胞因子等的按需生产,从而在传染病预防、肿瘤免疫治疗和罕见病/慢性病蛋白替代三大方向同步突破。
依托这一高度可编程的平台优势,mRNA技术的应用边界正迅速拓宽,研发重心已从单一病毒防护向构建全面的医疗应用延伸。在传染病预防领域,mRNA平台已拓展至结核等难防病原体,例如美国哈佛大学通过挖掘CD4 T细胞抗原库开发了效果超越卡介苗的新型结核病三价mRNA疫苗[31]。在复杂疾病治疗方面,mRNA作为临时体内工厂展现出独特优势,美国博德研究所等利用mRNA技术使老年小鼠肝细胞表达关键免疫信号DLL1、FLT3L和IL-7,从而促进新生免疫细胞产生,提升衰老个体的免疫应答和抗肿瘤能力[32]。面向更广泛的慢病管理需求,日本大阪大学等机构开发了一种由三个mRNA组成的新型智能响应型mRNA系统,能响应体内多种疾病标志物,自主动态调节蛋白表达[33],表明mRNA药物正迈向动态精准治疗的新阶段。2.2.1 预防性mRNA疫苗的传染病预防临床进展
在预防性疫苗方面,随着疫情形势变化,相关企业正积极将mRNA平台拓展至流感、RSV、带状疱疹等其他传染病领域,并从单一疫苗向联合疫苗迈进。2025年,该领域取得多项重要进展。美国辉瑞公司的四价流感mRNA疫苗在Ⅲ期临床试验中证实其效果优于传统灭活疫苗[34]。美国Moderna公司开发的COVID-19与流感联合疫苗mRNA-1083,在Ⅲ期试验中显示对四种病毒株提升20%~40%的免疫应答[35]。针对带状疱疹,深信生物、艾博生物等多家国内企业开发的mRNA疫苗已进入临床Ⅱ期,部分采用冻干工艺提升了储运便利性。在攻克艾滋病毒(HIV)的难题上,美国斯克里普斯研究所通过Ⅰ期临床试验验证,利用分阶段mRNA疫苗策略可有效引导人体产生广谱中和抗体,为开发有效的预防疫苗开辟了新路径[36]。2.2.2 mRNA肿瘤疫苗的抗肿瘤治疗临床进展
随着技术平台趋于成熟,mRNA肿瘤疫苗已成为后疫情时代极具前景的应用方向,主要分为个体化与通用型两类(表3)。
个体化mRNA疫苗旨在激发持久且特异性的抗肿瘤免疫。由美国Moderna公司与默沙东联合开发的mRNA-4157进展最为显著,已进入Ⅲ期临床。其Ⅱ期数据显示,与帕博利珠单抗联用可使高危黑色素瘤术后患者的2.5年总生存率达到96%,该疫苗的临床适应证也已拓展至肾细胞癌、尿路上皮癌等多个癌种。德国BioNTech公司开发的个体化疫苗Cevumeran(BNT122)在一项临床研究中显示,与化疗及阿替利珠单抗联用后,能产生疫苗诱导T细胞反应的胰腺癌患者,其长期无复发生存期显著优于无反应者[37]。
在通用型疫苗的研发中,进度最快的候选药物也已推进至临床后期,例如德国BioNTech公司靶向HPV E6/E7的疫苗BNT113,目前正处于Ⅱ/Ⅲ期临床阶段。
表3进入临床阶段的mRNA肿瘤疫苗研发情况
药品名称
研发机构
抗原类型/靶点
疾病
全球最高研发阶段
Intismeran autogene(mRNA-4157)
美国默沙东、美国Moderna公司
个体化抗原
非小细胞肺癌、黑色素瘤、肾细胞癌、尿路上皮癌、皮肤鳞状细胞癌、非肌层浸润性膀胱癌
Ⅲ期临床
Cevumeran(BNT122)
德国BioNTech公司
个体化抗原
黑色素瘤、结直肠癌/胰导管腺癌
Ⅱ期临床
GRANITE-001
美国Gritstone公司
个体化抗原
非小细胞肺癌、尿路上皮癌、结直肠癌、胃食管癌
Ⅱ/Ⅲ期临床
奥基司米瑞
瑞士罗氏、德国BioNTech公司
个体化抗原
非小细胞肺癌、三阴性乳腺癌、胰腺癌、头颈癌、黑色素瘤、肾癌/膀胱癌、尿路上皮癌、结直肠癌
Ⅱ期临床
LK101
立康生命科技
个体化抗原
非小细胞肺癌、小细胞肺癌、肝细胞癌
Ⅱ期临床
iNeo-Vac-R01
纽安津
个体化抗原
肝内胆管癌、消化道癌症
Ⅰ/Ⅱ期临床
CVGBM
CureVac(德国BioNTech公司旗下)
个体化抗原
胶质母细胞瘤、星形细胞瘤
Ⅰ/Ⅱ期临床
BNT113
德国BioNTech公司
通用型肿瘤相关抗原 (HPV E6、HPV E7)
HPV-16阳性头颈癌
Ⅱ/Ⅲ期临床
BNT111
德国BioNTech公司
通用型肿瘤相关抗原(NY-ESO-1、MAGE-A3、酪氨酸酶、TPTE)
黑色素瘤
Ⅱ期临床
CV9202
CureVac(德国BioNTech公司旗下)、德国勃林格殷格翰
通用型肿瘤相关抗原(NY-ESO-1、MAGEC2、survivin、Muc1、5T4、MAGEC1)
非小细胞肺癌
Ⅱ期临床
BNT116
美国再生元、德国BioNTech公司
通用型肿瘤相关抗原(MAGEA3、MAGEA4、CLDN6、PRAME、KKLC1、MAGEC1)
非小细胞肺癌
Ⅱ期临床
Gindameran
德国BioNTech公司
通用型肿瘤相关抗原(NY-ESO-1、MAGEA3、TYR、TPTE)
黑色素瘤
Ⅱ期临床
mRNA-4359
美国Moderna公司
通用型免疫调节疫苗(PDL1、IDO)
实体瘤(黑色素瘤、非小细胞肺癌、非肌层浸润性膀胱癌、头颈部鳞状细胞癌、微卫星稳定结直肠癌、基底细胞癌、三阴性乳腺癌)
Ⅰ/Ⅱ期临床
AFN0328
安科生物、阿法纳生物
通用型肿瘤相关抗原
宫颈癌、肛门癌、宫颈上皮内瘤变、外阴癌、阴道癌、阴茎癌
Ⅰ/Ⅱ期临床
RG002
仁景生物
通用型肿瘤相关抗原
宫颈上皮内瘤变、HPV相关癌症、HPV引起癌前病变
Ⅰ/Ⅱ期临床
EI-201
比利时eTheRNA immunotherapies公司
通用型肿瘤相关抗原(HPV E6、HPV E7)
HPV相关癌症(宫颈癌、肛门癌、外阴癌、阴道癌、口咽癌、阴茎癌)
Ⅰ/Ⅱ期临床
EVM14
云顶新耀
通用型肿瘤相关抗原
头颈部鳞状细胞癌、鳞状非小细胞肺癌
Ⅰ/Ⅱ期临床
2.2.3 mRNA药物的罕见病和慢病治疗临床进展
mRNA药物通过在体内编码生成相应的酶、抗体、调节因子等蛋白质,正逐步在罕见病、肿瘤以及其他慢性病领域展现潜在优势。
在罕见遗传代谢病领域,mRNA作为一种体内酶替代疗法策略得到深入验证,解决了传统蛋白药物难以高效入胞的难题,多条管线已进入临床Ⅱ期(表4)。该领域的先驱美国Moderna公司布局了多款核心药物,其中用于治疗甲基丙二酸血症的mRNA-3705和用于丙酸血症的mRNA-3927均已进入Ⅰ/Ⅱ期临床,并初步验证了其安全有效性。
在肿瘤治疗领域,与mRNA肿瘤疫苗通过表达抗原激活抗肿瘤免疫反应不同,mRNA药物主要通过表达治疗性的双特异性抗体或免疫调节细胞因子,实现灵活可控的免疫治疗。如德国BioNTech公司的BNT142编码靶向CLDN6/CD3的双抗,以及美国Moderna公司的mRNA-2808编码靶向BCMA/CD3或GPRC5D/CD3的双抗,均在Ⅰ/Ⅱ期临床试验中探索用于实体瘤或多发性骨髓瘤的治疗。这种模块化设计便于快速迭代靶点,且mRNA的短暂表达特性有助于控制免疫毒性风险。同时,通过表达免疫刺激细胞因子来激活肿瘤微环境的策略也备受关注,如艾博生物的ABO2011与嘉晨西海的JCXH-211等药物,旨在重塑免疫应答。
在慢性病领域,局部递送型mRNA药物取得了突破性进展。进展最快的为瑞宏迪医药开发的RGL-2102,已进展至Ⅱ/Ⅲ期临床,这是全球首个获批临床的外用型mRNA药物,通过在伤口局部递送编码人肝细胞生长因子的mRNA,刺激血管新生和肉芽组织形成,加速愈合,为严重肢体缺血和糖尿病足等患者带来全新治疗思路。
表4 临床阶段的mRNA药物研发情况
药品名称
研发机构
靶点
疾病
全球最高研发阶段
ARCT-810
美国Arcturus公司、CureVac(德国BioNTech公司旗下)
OTC
鸟氨酸氨甲酰转移酶缺乏症
Ⅱ期临床
ARCT-032
美国Arcturus公司
CFTR
囊性纤维化
Ⅱ期临床
RCT-2100
美国ReCode Therapeutics公司
CFTR
囊性纤维化
Ⅰ/Ⅱ期临床
VX-522
美国福泰制药、美国Moderna公司
CFTR
囊性纤维化
Ⅰ/Ⅱ期临床
RCT-1100
美国ReCode Therapeutics公司
DNAI1
原发性纤毛运动障碍
Ⅰ/Ⅱ期临床
mRNA-3705
美国Moderna公司
MUT
甲基丙二酸血症
Ⅰ/Ⅱ期临床
mRNA-3927
美国Moderna公司
PCCA、PCCB
丙酸血症
Ⅰ/Ⅱ期临床
mRNA-3283
美国Moderna公司
PH4H
苯丙酮尿症
Ⅰ/Ⅱ期临床
RTT-1
日本Grann Pharmaceuticals公司
-e
瑞特综合征
Ⅰ期临床
SPOT-mRNA03
思珀诺因
dystrophin
假肥大性肌营养不良
Ⅰ期临床
DSL101
星核迪赛
ATP7B
威尔逊病
Ⅰ期临床
mRNA-2808
美国Moderna公司
BCMA、CD3、GPRC5D、FCRL5
多发性骨髓瘤
Ⅰ/Ⅱ期临床
ABO2011
艾博生物
IL-12
实体瘤
Ⅱ期临床
BNT142
德国BioNTech公司
CD3、CLDN6
实体瘤
Ⅰ/Ⅱ期临床
STX-001
美国Strand Therapeutics公司
IL-12
实体瘤
Ⅰ/Ⅱ期临床
MTS105
剂泰医药
CD3、GPC3
肝细胞癌
Ⅰ期临床
JCXH-211
嘉晨西海
IL-12
实体瘤
Ⅰ期临床
PMC2129G12
远泰生物
CD3、EpCAM
EpCAM阳性的晚期恶性实体瘤(肝癌、结直肠癌、乳腺癌等)
Ⅰ期临床
mRNA-4203
美国Moderna公司、荷兰Immatics公司
PRAME
黑色素瘤、滑膜肉瘤
Ⅰ期临床
RGL-2102
瑞宏迪医药
HGF
糖尿病足
Ⅱ/Ⅲ期临床
ETH47
德国Ethris公司
IFNλ
病毒感染、哮喘
Ⅱ期临床
VMB-100
瑞士Versameb公司
IGF-1
压力性尿失禁、混合性尿失禁
Ⅱ期临床
2.3 环状RNA
环状RNA作为新兴技术的潜力正在被加速发掘,其功能涵盖了蛋白编码与非编码功能调控,基于多重功能机制的疫苗/药物潜力不断挖掘,为临床应用奠定了坚实基础。在蛋白质编码表达功能方面,环状RNA与mRNA相似,均可作为预防性疫苗、治疗性疫苗及治疗性药物发挥作用。有研究发现通过关节内局部递送编码RNA结合蛋白Musashi2的环状RNA,能够有效逆转骨关节炎的病理状态[38]。与传统mRNA及普通环状RNA疫苗相比,基于小环状RNA开发的新型癌症疫苗可有效避免蛋白激酶R激活,实现了低毒、10倍强效且长效的抗原特异性T细胞反应[39]。值得关注的是,中国科学院分子细胞科学卓越创新中心基于环状RNA的蛋白质支架特性,开发出一种环形RNA适配体,通过抑制蛋白激酶R异常激活来治疗阿尔茨海默病[40]。
随着技术平台的成熟,环状RNA药物已初步进入临床验证阶段。目前,国内企业在临床推进进度上处于国际前列(表5)。当前临床阶段的环状RNA主要基于蛋白质编码机制,应用方向覆盖了预防性疫苗、治疗性疫苗和治疗性药物,其中治疗性药物的开发最为活跃。转录本生物的RXRG001是全球首个获美国FDA许可进入临床试验的环状RNA药物,用于治疗放射性口干症,目前已完成Ⅰ/Ⅱ期临床试验的首例患者给药。环码生物围绕心血管疾病布局了多款产品,HM2002通过编码血管内皮生长因子促进心肌血管新生,用于治疗缺血性心脏病/缺血性心力衰竭,另一款HM2003用于慢性肢体威胁性缺血的治疗,已获FDA孤儿药资格认定。圆因生物的TI‑0093通过表达HPV E6/E7抗原激活免疫系统治疗肿瘤,是全球首个在肿瘤治疗领域开展临床研究的环状RNA药物。
表5临床阶段的环状RNA药物研发情况
类型
药品名称
研发机构
靶点
疾病
全球最高研发阶
治疗性药物
RXRG001
转录本
AQP1
放射引起的口腔干燥
Ⅰ/Ⅱ期临床
治疗性药物
HM2002
环码生物
VEGF
冠心病、缺血性心力衰竭
Ⅰ期临床
治疗性药物
HM2003
环码生物
-
肢体缺血性疾病
Ⅰ期临床
治疗性疫苗
TI-0093
圆因生物
HPV E6/HPV E7
实体瘤、宫颈癌、头颈部鳞状细胞癌、HPV相关肿瘤
Ⅰ期临床
预防性疫苗
TI-0010
圆因生物
SARS-CoV-2
新型冠状病毒感染
Ⅰ期临床
3 RNA疗法的产业化进展
随着核心技术突破与临床价值确证,小核酸药物产业正从技术验证迈向规模化商业拓展,多款产品持续彰显其在罕见病与慢性病领域的临床价值和商业潜力,全球市场高速增长。如治疗脊髓性肌萎缩症的首款药物诺西那生钠自2016年上市后销售额迅速增长,长期位居小核酸药物销售首位。降脂药英克司兰钠凭借“一年两针”的长效优势,销售额从2021年的1 200万美元激增至2024年的7.54亿美元,正迅速跻身“重磅炸弹”药物行列。
而高额的战略合作与并购也推动着产业格局重塑,2025年,RNA疗法领域的交易热度空前,各大跨国巨头如诺华、礼来、罗氏及GSK等纷纷通过授权或收购的方式获得各平台技术以及潜在的临床管线权益等(图2)。研发竞争核心聚焦递送技术与适应证拓展。在平台技术方面,肝外组织递送技术是当前合作的热门焦点。如圣因生物的组织选择性递送技术LEAD(ligand and enhancer assisted delivery,新型配体与增效基团协同递送)平台,美国Avidity公司的AOC平台,美国Manifold Bio公司合作开发AI驱动的脑部递送技术,以及韩国ABL Bio公司用于神经退行性疾病研究的血脑屏障穿梭平台等。在研发管线方面,合作大多集中于siRNA药物与ASO药物,适应证以降脂降压等心血管代谢疾病和中枢神经系统疾病为主。
图2 2025年RNA疗法领域重要技术平台/研发管线的交易/合作
值得一提的是,中国的创新力量在其中正成为关键技术发源地。舶望制药、圣因生物、迈威生物等已成为国际巨头如诺华、礼来、罗氏等的合作伙伴,且交易金额已达到国际水平,最高的潜在总金额高达52亿美元。从交易内容看,主要以siRNA的药物研发管线为主,覆盖心血管、代谢、神经系统等多个疾病领域。圣因生物的LEAD肝外递送平台因其在解决肝外组织靶向递送这一行业核心难题上的巨大潜力,成为吸引高额交易的关键技术资产。4 展望
作为引领生物医药范式变革的战略性前沿,RNA疗法凭借其精准靶向与灵活设计的优势,初步展现了在遗传病、传染病及肿瘤防治领域的突破性潜力。但递送效率、组织选择性及长期安全性仍是核心瓶颈。未来,该领域将通过关键技术突破、基础研究深化、临床应用拓展以及与AI的深度融合,实现从“可及治疗”向“精准长效治疗”的范式跨越。
一是关键技术突破聚焦肝外递送与智能修饰。针对脑、肺、肿瘤等难治部位的靶向递送系统将成为研发核心,通过配体修饰LNP或开发新型载体,显著提升RNA在特定组织的富集。同时,RNA化学修饰技术正从基础优化迈向智能精准调控,实现对病灶的时空特异性激活与按需释放,并发掘具有新功能的修饰分子。
二是基础科学的深化将解锁全新的药物机制与应用范畴。系统解析疾病在RNA层面的致病机制,利用多组学技术发掘可干预RNA序列,以拓展疗法适应证。同时,深入揭示非编码RNA功能、RNA修饰调控规律及分子结构与稳定性之间的内在关联,为设计下一代高效、低免疫原性的RNA治疗分子提供根本指导。
三是临床应用生态将呈现多元化与长效化发展。随着技术成熟,更多遗传性罕见病患者将实现从无到有、从有到优的治疗;长效RNA疗法有望从根本上改善心血管代谢等慢性病患者的生活质量,减轻医疗体系负担;mRNA肿瘤疫苗将与CAR-T疗法、免疫检查点抑制剂等联合,成为构建肿瘤领域多维度免疫治疗体系的关键支柱。
四是人工智能深度赋能研发全链条。AI技术将贯穿从序列设计、载体筛选到药效预测的全过程,提升开发效率与成功率,缩短研发周期。
最终,随着前沿科学、颠覆技术、敏捷监管与可持续市场协同形成良性生态,将激发RNA技术的巨大科学潜力,引领医学进入可编程、精准化与一体化和技术普惠的新时代。
收稿日期:2026-01-09 修回日期:2026-02-06
基金项目:国家实验室专项项目(GZNL2024A01026)
*通信作者:E-mail:lizhenqi@sinh.ac.cn
李祯祺,硕士,中国科学院上海营养与健康研究所(中国科学院上海生命科学信息中心) 副研究馆员,产业情报与区域发展研究中心副主任。主要从事生命科学与生物技术领域的情报研究工作。作为课题/子课题负责人或项目骨干/高级研究人员参与国家重点研发计划等项目20余项;撰写中英文科技论文近40篇,参编国家部委和重要智库机构的专著30余册。先后获得华东科技情报成果奖6项、上海科技情报成果奖8项,以及华东科技情报优秀工作者、上海科技情报优秀工作者称号等荣誉。
参考文献
[1]Krawczyk PS, Mazur M, Orzeł W, et al. Re-adenylation by TENT5A enhances efficacy of SARS-CoV-2 mRNA vaccines. Nature, 2025, 641: 984-92.
[2] Fukuchi K, Nakashima Y, Abe N, et al. Internal cap-initiated translation for efficient protein production from circular mRNA. Nat Biotechnol, 2026, 44: 120-32.
[3] Zhang H, Liu H, Xu Y, et al. Deep generative models design mRNA sequences with enhanced translational capacity and stability. Science, 2025, 390: eadr8470.
[4] Mo O, Zhang Z, Cheng X, et al. mRNA designer: an integrated web server for optimizing mRNA design and protein translation in eukaryotes. Nucleic Acids Res, 2025, 53(W1): W415-26.
[5] Chiba S, Lim KRQ, Sheri N, et al. eSkip-Finder: amachine learning-based web application and database to identify the optimal sequences of antisense oligonucleotides for exon skipping. Nucleic Acids Res, 2021, 49(W1): W193-98.
[6] Hwang G, Kwon M, Seo D, et al. ASOptimizer: Optimizing antisense oligonucleotides through deep learning for IDO1 gene regulation. Mol Ther Nucleic Acids, 2024, 35: 102186.
[7] Tarbashevich K, Ghosh A, Das A, et al. Optochemical control over mRNA translation by photocaged phosphorodiamidate morpholino oligonucleotidesin vivo. Nat Commun, 2025, 16: 3614.
[8] Zhao X, Xu J, Liang X, et al. NQO1-activatable circular antisense oligonucleotides for tumor-cell-specific survivin gene silencing and antitumor therapy. J Med Chem, 2025, 68: 4466-76.
[9] Bérouti M, Wagner M, Greulich W, et al. Pseudouridine RNA avoids immune detection through impaired endolysosomal processing and TLR engagement. Cell, 2025, 188: 4880-95.e15.
[10] Kim M, Pyo Y, Hyun SI, et al. Exogenous RNA surveillance by proton-sensing TRIM25. Science, 2025, 388: eads4539.
[11] Iwai H, Kimura Y, Honma M, et al. Position-specific ORF nucleoside-ribose modifications enabled by complete chemical synthesis enhance mRNA stability and translation. Nat Commun, 2025, 16: 9995.
[12]Castaño D, Bettini E, Kumar B, et al. Distinct components of mRNA vaccines cooperate to instruct efficient germinal center responses. Cell, 2025, 188: 7461-80.e23.
[13] Liu Y, Liu Q, Zhang B, et al. Generation of tolerogenic antigen-presenting cells in vivo via the delivery of mRNA encoding PDL1 within lipid nanoparticles. Nat Biomed Eng, 2025, 9: 1320-34.
[14] Rudra A, Gupta A, Reed K, et al. Degradable cyclic amino alcohol ionizable lipids as vectors for potent influenza mRNA vaccines. Nat Nanotechnol, 2025, 20: 1831-42.
[15]Chan A, Kirtane AR, Qu QR,et al. Designing lipid nanoparticles using a transformer-based neural network. Nat Nanotechnol, 2025, 20: 1491-501.
[16]Wang C, Xue Y, Markovic T, et al. Blood-brain-barrier-crossing lipid nanoparticles for mRNA delivery to the central nervous system. Nat Mater, 2025, 24: 1653-63.
[17]Cao D, Hou X, Wang C, et al. Lipid nanoparticles for mRNA delivery in brain via systemic administration. Sci Adv, 2025, 11: eadw0730.
[18] Xue Y, Hou X, Wang S, et al. Antimicrobial peptide delivery to lung as peptibody mRNA in anti-inflammatory lipids treats multidrug-resistant bacterial pneumonia. Nat Biotechnol, 2025:10.1038/s41587-025-02928-x.
[19] Quijano E, Martinez-Saucedo D, Ianniello Z, et al. Systemic administration of an RNA binding and cell-penetrating antibody targets therapeutic RNA to multiple mouse models of cancer. Sci Transl Med, 2025, 17: eadk1868.
[20] Means JC, Martinez-Bengochea AL, Louiselle DA, et al. Rapid and scalable personalized ASO screening in patient-derived organoids. Nature, 2025, 638: 237-43.
[21]Borges B, BrownSM, Chen WJ, et al. Intra-amniotic antisense oligonucleotide treatment improves phenotypes in preclinical models of spinal muscular atrophy. Sci Transl Med, 2025, 17: eadv4656.
[22]Tao Y, Tian C, Qi S, et al. Targeting both death and paracaspase domains of MALT1 with antisense oligonucleotides overcomes resistance to immune-checkpoint inhibitors. Nat Cancer, 2025, 6: 702-17.
[23] Hipp JF, Bacino CA, Bird LM, et al. The UBE3A-ATS antisense oligonucleotide rugonersen in children with Angelman syndrome: a phase 1 trial. Nat Med, 2025, 31: 2936-45.
[24]Wagner M, Berecki G, Fazeli W, et al. Antisense oligonucleotide treatment in a preterm infant with early-onset SCN2A developmental and epileptic encephalopathy. Nat Med, 2025, 31: 2174-78.
[25] Komaki H, Takeshita E, Kunitake K, et al. Phase 1/2 trial of brogidirsen: Dual-targeting antisense oligonucleotides for exon 44 skipping in Duchenne muscular dystrophy. Cell Rep Med, 2025, 6:101901.
[26] Rosenson RS, López JAG, Gaudet D, et al. Olpasiran, oxidized phospholipids, and systemic inflammatory biomarkers: Results from the OCEAN(a)-DOSE trial. JAMA Cardiol, 2025, 10: 482-86.
[27] Nissen SE, Ni W, Shen X, et al. Lepodisiran - a long-duration small interfering RNA targeting lipoprotein(a). N Engl J Med, 2025, 393: 411
[28]Ray KK, Oru E, Rosenson RS, et al. Durability and efficacy of solbinsiran, aGalNAc-conjugated siRNA targeting ANGPTL3, in adults with mixed dyslipidaemia (PROLONG-ANG3): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet, 2025, 28: S0140-6736(25)00507-0.
[29]Desai AS, Karns AD, Badariene J, et al. Add-on treatment with zilebesiran for inadequately controlled hypertension: The KARDIA-2 randomized clinical trial. JAMA, 2025, 334: 46-55.
[30] Loomba R, Morgan E, Yousefi K, et al. Antisense oligonucleotide DGAT-2 inhibitor, ION224, for metabolic dysfunction-associated steatohepatitis (ION224-CS2): results of a 51-week, multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet, 2025, 406: 821-31.
[31]Vidal SJ, Lasrado N, Tostanoski LH, et al. Mining the CD4 antigen repertoire for next-generation tuberculosis vaccines. Cell, 2025, 188: 6791-03.e13.
[32]Friedrich MJ, Pham J, Tian J, et al. Transient hepatic reconstitution of trophic factors enhances aged immunity. Nature, 2026, 650: 481-89.
[33] Nakanishi H, Itaka K. Extracellular ligand-responsive translational regulation of synthetic mRNAs using engineered receptors. NPG Asia Mater, 2025, 17. doi: 10.1038/s41427-025-00607-6
[34] Fitz-Patrick D, McVinnie DS, Jackson LA, et al. Efficacy, immunogenicity, and safety of modified mrna influenza vaccine. N Engl J Med, 2025, 393: 2001-11.
[35]Rudman Spergel AK, Wu I, Deng W, et al. Immunogenicity and safety of influenza and COVID-19 multicomponent vaccine in adults ≥50 years: A randomized clinical trial. JAMA, 2025, 333: 1977-87.
[36]Willis JR, Prabhakaran M, Muthui M, et al. Vaccination with mRNA-encoded nanoparticles drives early maturation of HIV bnAb precursors in humans. Science, 2025, 389: eadr8382.
[37] Sethna Z, Guasp P, Reiche C, et al. RNA neoantigen vaccines prime long-lived CD8+ T cells in pancreatic cancer. Nature, 2025, 639: 1042-51.
[38]Suo J, Li L, Tan W, et al. Circular RNA-based protein replacement therapy mitigates osteoarthritis in male mice. Nat Commun, 2025, 16: 8480.
[39]Zhang Y, Liu X, Shen T, et al. Small circular RNAs as vaccines for cancer immunotherapy. Nat Biomed Eng, 2025, 9: 249-67.
[40]Feng X, Jiang BW, Zhai SN, et al. Circular RNA aptamers targeting neuroinflammation ameliorate Alzheimer disease phenotypes in mouse models. Nat Biotechnol, 2025. doi: 10.1038/s41587-025-02624-w.
原文刊登于《生命科学》2026年第38卷第02期
《生命科学》是由中国科学院上海营养与健康研究所主办,国家自然科学基金委员会生命科学部和中国科学院生命科学和医学学部共同指导的综合性学术期刊。1988年创刊,原刊名为《生物学信息》内部发行;1992年起更名为《生命科学》,公开发行CN31-1600/Q,大16开,96页。本刊是“中文核心期刊” “中国科技核心期刊” “中国科学引文数据库来源期刊(CSCD)”。
信使RNA核酸药物寡核苷酸siRNA疫苗
2026-02-12
− Achieved Fourth Quarter and Full Year 2025 Global Net Product Revenues of $995 Million and $2,987 Million, Respectively, Representing 121% and 81% Growth Compared to Same Periods in 2024 –
− Attained GAAP and non-GAAP Profitability for Full Year 2025, with Sustainable Growth in Operating Income Expected –
− Launched
“
Alnylam 2030
”
Strategy Focused on Scaling Alnylam through Durable ATTR Leadership, Long-Term Sustainable Innovation, and Exceptional Financial Results –
− Announced 2026 Pipeline Goals, Including 4 Clinical Readouts, 3 Ongoing Pivotal Studies, 3 Phase 2 Study Initiations, and 3+ New IND Filings –
− Reiterates Net Product Sales Guidance and Provides Additional 2026 Financial Guidance –
CAMBRIDGE, Mass.--(BUSINESS WIRE)--
Alnylam Pharmaceuticals, Inc.
(Nasdaq: ALNY), the leading RNAi therapeutics company, today reported its consolidated financial results for the fourth quarter and full year ended December 31, 2025 and reviewed recent business highlights.
“2025 was a year of key accomplishments for Alnylam, highlighted by the landmark approval of AMVUTTRA for ATTR-CM in the U.S., which drove total net product revenues of nearly $3 billion, or 81% growth year-over-year, and propelled us to profitability. We also achieved great progress across our portfolio, initiating three Phase 3 studies, expanding our pipeline with four proprietary CTAs, and launching a potential best-in-class enzymatic ligation-based RNAi manufacturing platform,” said Yvonne Greenstreet, M.D., Chief Executive Officer of Alnylam. “Further, we are excited to have recently unveiled our new set of five-year aspirational goals,
Alnylam 2030
, under which we aim to achieve global TTR leadership with a durable franchise; grow through innovation by delivering therapies that prevent, halt, or reverse disease; and scale with financial discipline and agility. By pursuing these ambitious goals, we believe Alnylam will drive substantial patient impact by addressing serious unmet medical needs around the world and create substantial long-term shareholder value.”
Fourth Quarter 2025 and Recent Significant Business Highlights
Total TTR: AMVUTTRA
®
(vutrisiran) & ONPATTRO
®
(patisiran)
Achieved global net product revenues for AMVUTTRA and ONPATTRO for the fourth quarter of $827 million and $32 million, respectively, together representing 151% total TTR growth compared to Q4 2024, and full year 2025 revenues of $2,314 million and $173 million, respectively, together representing 103% total TTR growth compared to full year 2024.
Presented new analyses from the HELIOS-B Phase 3 clinical trial of
vutrisiran
in patients with ATTR-CM at the
American Heart Association Scientific Sessions 2025
.
Cardiovascular magnetic resonance (CMR) and echocardiographic analyses demonstrated that treatment with vutrisiran resulted in significant changes indicating significant improvement on multiple functional and structural cardiac parameters.
CMR imaging showed amyloid regression in 22% of vutrisiran treated patients with no regression found in patients who received placebo.
Treatment with vutrisiran preserved kidney function in HELIOS-B patients, and reduced risk of death and cardiovascular events in patients with advanced chronic kidney disease.
Total Rare: GIVLAARI
®
(givosiran) & OXLUMO
®
(lumasiran)
Achieved global net product revenues for GIVLAARI and OXLUMO for the fourth quarter of $87 million and $50 million, respectively, together representing 26% total Rare growth compared to Q4 2024, and full year 2025 revenues of $308 million and $191 million, respectively, together representing $500 million in revenues and 18% total Rare growth compared to full year 2024.
Other Highlights
Initiated a Phase 2 clinical trial of
ALN-4324
, an investigational RNAi therapeutic targeting GRB14 for type 2 diabetes mellitus.
Initiated a Phase 1 clinical trial of
ALN-2232
, an investigational RNAi therapeutic targeting ACVR1C in adipose tissue for obesity and weight management.
Advanced two new programs (
ALN-4285
and
ALN-4915
) into clinical development in healthy volunteers.
Announced the planned expansion of its state-of-the-art manufacturing facility in Norton, Massachusetts. The Company plans to invest $250 million to develop the industry's first fully dedicated, proprietary siRNA enzymatic-ligation manufacturing facility. This new enzymatic-ligation platform, siRELIS™, is expected to meaningfully expand capacity, significantly reduce production costs, and position the Company to support future launches across its growing pipeline of potential new medicines.
Additional Business Updates
Announced changes to the Company's Board of Directors, including the departures of Mike Bonney and Carolyn Bertozzi, Ph.D., and the appointment of Stuart Arbuckle.
Key Upcoming Events
The Company will host an investor webinar marking the one-year anniversary of the FDA approval of AMVUTTRA in ATTR-CM on March 24, 2026. The Company will highlight progress in delivering for ATTR-CM patients and the long-term growth and durability of its flagship TTR franchise.
In the first half of 2026, Alnylam expects to:
Complete enrollment in the cAPPricorn-1 Phase 2 clinical trial of
mivelsiran
in patients with cerebral amyloid angiopathy.
Initiate a Phase 2 clinical trial of
mivelsiran
in patients with Alzheimer's disease.
Initiate a Phase 2 clinical trial of
ALN-6400
in a second bleeding disorder.
In the second half of 2026, Alnylam expects to announce clinical de-risking data from several pipeline programs, including:
Results from Phase 1 and Phase 2 clinical trials of
ALN-6400
in healthy volunteers and patients with hereditary hemorrhagic telangiectasia (HHT), respectively.
Results from a Phase 1 clinical trial of
ALN-HTT02
in patients with Huntington's disease.
Results from a Phase 1 clinical trial of
ALN-2232
in obesity and weight management.
Financial Results for the Fourth Quarter and Full Year 2025
Three Months Ended December 31,
Twelve Months Ended December 31,
(In thousands, except per share amounts)
2025
2024
2025
2024
Total revenues
$
1,097,033
$
593,166
$
3,713,937
$
2,248,243
GAAP Income (loss) from operations
$
131,718
$
(105,159
)
$
501,578
$
(176,885
)
Non-GAAP Income (loss) from operations
$
203,350
$
(13,514
)
$
849,813
$
95,199
GAAP Net income (loss)
$
111,543
$
(83,763
)
$
313,747
$
(278,157
)
Non-GAAP Net income (loss)
$
169,753
$
8,048
$
683,644
$
(3,051
)
GAAP Net income (loss) per common share — basic
$
0.84
$
(0.65
)
$
2.39
$
(2.18
)
GAAP Net income (loss) per common share — diluted
$
0.82
$
(0.65
)
$
2.33
$
(2.18
)
Non-GAAP Net income (loss) per common share — basic
$
1.28
$
0.06
$
5.22
$
(0.02
)
Non-GAAP Net income (loss) per share — diluted
$
1.25
$
0.06
$
5.08
$
(0.02
)
For an explanation of our use of non-GAAP financial measures, refer to the “Use of Non-GAAP Financial Measures” section later in this press release and for a reconciliation of each non-GAAP financial measure to the most comparable GAAP measure, see the tables at the end of this press release.
Revenue Summary
Three Months Ended December 31,
(In thousands, except percentages)
2025
2024
% Change
At CER*
Net product revenues:
AMVUTTRA
$
826,588
$
286,510
189
%
187
%
ONPATTRO
31,687
56,103
(44
)%
(45
)%
Total TTR net product revenues
858,275
342,613
151
%
149
%
GIVLAARI
86,796
64,645
34
%
32
%
OXLUMO
49,646
43,573
14
%
10
%
Total Rare net product revenues
136,442
108,218
26
%
23
%
Total net product revenues
994,717
450,831
121
%
119
%
Net revenues from collaborations:
Roche
32,954
12,014
174
%
174
%
Regeneron Pharmaceuticals
7,834
30,657
(74
)%
(74
)%
Novartis AG
—
60,003
(100
)%
(100
)%
Other
155
4,274
(96
)%
(96
)%
Total net revenues from collaborations
40,943
106,948
(62
)%
(62
)%
Royalty revenue
61,373
35,387
73
%
73
%
Total revenues
$
1,097,033
$
593,166
85
%
83
%
* Change at constant exchange rates, or CER, represents percent change calculated as if exchange rates had remained unchanged from those used during the three months ended December 31, 2024. CER is a non-GAAP financial measure.
Twelve Months Ended December 31,
(In thousands, except percentages)
2025
2024
% Change
At CER*
Net product revenues:
AMVUTTRA
$
2,313,836
$
970,450
138
%
137
%
ONPATTRO
172,789
252,857
(32
)%
(32
)%
Total TTR net product revenues
2,486,625
1,223,307
103
%
102
%
GIVLAARI
308,487
255,871
21
%
20
%
OXLUMO
191,437
167,050
15
%
13
%
Total Rare net product revenues
499,924
422,921
18
%
17
%
Total net product revenues
2,986,549
1,646,228
81
%
80
%
Net revenues from collaborations:
Roche
394,881
119,489
230
%
230
%
Regeneron Pharmaceuticals
113,957
302,798
(62
)%
(62
)%
Novartis AG
—
79,759
(100
)%
(100
)%
Other
44,528
8,175
445
%
445
%
Total net revenues from collaborations
553,366
510,221
8
%
8
%
Royalty revenue
174,022
91,794
90
%
90
%
Total revenues
$
3,713,937
$
2,248,243
65
%
64
%
* Change at constant exchange rates, or CER, represents growth calculated as if exchange rates had remained unchanged from those used during the twelve months ended December 31, 2024. CER is a non-GAAP financial measure.
Total Net Product Revenues
Net product revenues increased 121% and 81% at actual currency during the three and twelve months ended December 31, 2025, respectively, compared to the same periods in 2024, and 119% and 80% at CER, respectively. The increases were primarily due to growth from AMVUTTRA revenues driven by increased patient demand, mainly in patients with ATTR-CM in the U.S., which was partially offset by a decreased number of patients on ONPATTRO, and due to growth from an increased number of patients on GIVLAARI and OXLUMO.
Net Revenues from Collaborations
Net revenues from collaborations decreased $66 million during the three months ended December 31, 2025, as compared to the same period in 2024, primarily due to revenue recognized under our license agreement with Novartis associated with the achievement of a specified Leqvio commercialization milestone during the three months ended December 31, 2024.
Net revenues from collaborations increased by $43 million during the twelve months ended December 31, 2025, as compared to the same period in 2024, primarily driven by recognition of $300 million of milestone revenue under our collaboration with Roche in September 2025 associated with the dosing of the first patient in the ZENITH Phase 3 clinical trial of zilebesiran and recognition of a $30 million payment in connection with the amendment to our agreement with Vir Biotechnology in March 2025. In comparison, during 2024, we recognized $185 million in revenues under our collaboration with Regeneron as we modified the collaboration in June 2024 and provided Regeneron with an exclusive license to develop, manufacture and commercialize cemdisiran as a monotherapy, and also recognized $65 million of milestone revenue under our collaboration with Roche in March 2024 associated with the dosing of the first patient in the KARDIA-3 Phase 2 clinical trial of zilebesiran.
Royalty Revenue
Royalty revenue increased during the three and twelve months ended December 31, 2025, as compared to the same periods in 2024, due to increased volume and rate of royalties earned from global net sales of Leqvio by Novartis.
Operating Expense Summary
Three Months Ended December 31,
Twelve Months Ended December 31,
(In thousands, except percentages)
2025
2024
% Change
2025
2024
% Change
Cost of goods sold
$
267,723
$
102,649
161
%
$
677,166
$
306,513
121
%
% of net product revenues
26.9
%
22.8
%
22.7
%
18.6
%
Cost of collaborations and royalties
$
—
$
168
(100
)%
$
4,705
$
16,857
(72
)%
GAAP Research and development expenses
$
372,218
$
300,169
24
%
$
1,319,775
$
1,126,232
17
%
Non-GAAP Research and development expenses
$
340,898
$
259,544
31
%
$
1,166,380
$
998,483
17
%
GAAP Selling, general and administrative expenses
$
325,374
$
295,339
10
%
$
1,210,713
$
975,526
24
%
Non-GAAP Selling, general and administrative expenses
$
285,062
$
244,319
17
%
$
1,015,873
$
831,191
22
%
Cost of Goods Sold
Cost of goods sold as a percentage of net product revenues increased during the three and twelve months ended December 31, 2025, as compared to the same periods in 2024, primarily as a result of increased sales of AMVUTTRA and an associated increase in the blended royalty rate payable on net sales of AMVUTTRA.
Research & Development (R&D) Expenses
GAAP and non-GAAP R&D expenses increased during the three and twelve months ended December 31, 2025, as compared to the same periods in 2024, primarily due to increased clinical trial expenses for the ZENITH Phase 3 clinical trial of zilebesiran, the TRITON-CM Phase 3 clinical trial of nucresiran in patients with ATTR-CM and the TRITON-PN Phase 3 clinical trial of nucresiran in patients with hATTR-PN, as well as increased employee compensation and related expenses to support our research and development pipeline and development expenses. Additionally, GAAP R&D expenses increased due to higher stock-based compensation expenses.
Selling, General & Administrative (SG&A) Expenses
GAAP and non-GAAP SG&A expenses increased during the three and twelve months ended December 31, 2025, as compared to the same periods in 2024, primarily due to higher employee compensation costs, including stock-based compensation, mainly driven by higher headcount, and increased marketing investment associated with the commercial launch of AMVUTTRA in ATTR-CM.
Other Financial Highlights
Interest expense
Interest expense for the three and twelve months ended December 31, 2025 of $65 million and $253 million, respectively, included interest of $38 million and $150 million, respectively, attributed to the liability related to the sale of future Leqvio royalties, and $25 million and $89 million, respectively, attributed to the liabilities related to the vutrisiran and zilebesiran development funding.
Benefit from (provision for) income taxes
During the three months year ended December 31, 2025, we recorded a benefit from income taxes of $25 million primarily related to the generation of Switzerland deferred tax assets, partially offset by additional U.S state income tax. During the twelve months ended December 31, 2025, we recorded a provision for income taxes of $9 million primarily related to U.S state income taxes, utilization of Switzerland net deferred tax assets, as well as taxable income from jurisdictions in which we are subject to tax. We will continue to utilize deferred tax assets in Switzerland to offset current cash tax liabilities and will continue to monitor the requirement for a valuation allowance against our net deferred tax assets in the U.S. and certain deferred tax assets in Switzerland.
Financial position
Cash, cash equivalents and marketable securities were $2.91 billion as of December 31, 2025, as compared to $2.69 billion as of December 31, 2024, with the increase primarily due to improved operating performance, proceeds from exercise of stock options, and net proceeds from the issuance of our 0.00% convertible senior notes due 2028, offset in part by cash paid for the partial repurchases of our 1.00% convertible senior notes due 2027.
Net cash provided by operating activities for the three and twelve months ended December 31, 2025 included $23 million and $118 million, respectively, of payments associated with the liability related to the sale of future Leqvio royalties recorded to interest expense, as well as $30 million and $85 million, respectively, of payments associated with the liabilities related to vutrisiran and zilebesiran development funding recorded to interest expense.
A reconciliation of our GAAP to non-GAAP results for the three and twelve months ended December 31, 2025 and 2024, is included in the tables at the end of this press release.
2026 Financial Guidance
Full-year 2026 financial guidance is summarized below:
Total TTR net product revenues (AMVUTTRA, ONPATTRO)
1
$4,400 million - $4,700 million
Total Rare net product revenues (GIVLAARI, OXLUMO)
1
$500 million - $600 million
Total net product revenues
1
$4,900 million - $5,300 million
Net product revenues growth vs. 2025 at currency exchange rates as of December 31, 2025
2
64% - 77%
Net product revenues growth vs. 2025 at constant exchange rates
2
64% - 77%
Net revenues from collaborations and royalties
$400 million - $500 million
Non-GAAP R&D and SG&A expenses
3
$2,700 million - $2,800 million
1
Full-year 2026 guidance utilizing currency exchange rates as of December 31, 2025: 1 EUR = 1.17 USD and 1 USD = 157 JPY
2
Representing growth calculated as if the exchange rates had remained unchanged from those used in 2025, which is a non-GAAP financial measure
3
Excludes $300 million - $400 million of stock-based compensation expense from estimated GAAP R&D and SG&A expenses
Use of Non-GAAP Financial Measures
This press release contains non-GAAP financial measures, including expenses adjusted to exclude certain non-cash expenses and non-recurring gains or losses outside the ordinary course of the Company’s business. These measures are not in accordance with, or an alternative to, GAAP, and may be different from non-GAAP financial measures used by other companies.
The items included in GAAP presentations but excluded for purposes of determining non-GAAP financial measures for the periods presented in this press release are stock-based compensation expenses, loss related to convertible debt, and realized and unrealized gains or losses on marketable equity securities. The Company has excluded the impact of stock-based compensation expense, which may fluctuate from period to period based on factors including the variability associated with performance-based grants for stock options and restricted stock units and changes in the Company’s stock price, which impacts the fair value of these awards. The Company has excluded the loss related to convertible debt because the Company believes the item is a non-recurring transaction outside the ordinary course of the Company’s business. The Company has excluded the impact of the realized and unrealized gains or losses on marketable equity securities because the Company does not believe these adjustments accurately reflect the performance of the Company’s ongoing operations for the period in which such gains or losses are reported, as their sole purpose is to adjust amounts on the balance sheet.
Percentage changes in revenue growth at CER are presented excluding the impact of changes in foreign currency exchange rates for investors to understand the underlying business performance. The current period’s foreign currency revenue values are converted into U.S. dollars using the average exchange rates from the prior period.
The Company believes the presentation of non-GAAP financial measures provides useful information to management and investors regarding the Company’s financial condition and results of operations. When GAAP financial measures are viewed in conjunction with non-GAAP financial measures, investors are provided with a more meaningful understanding of the Company’s ongoing operating performance and are better able to compare the Company’s performance between periods. In addition, these non-GAAP financial measures are among those indicators the Company uses as a basis for evaluating performance, allocating resources and planning and forecasting future periods. Non-GAAP financial measures are not intended to be considered in isolation or as a substitute for GAAP financial measures. A reconciliation between GAAP and non-GAAP measures is provided later in this press release.
Conference Call Information
Management will provide an update on the Company and discuss fourth quarter and full year 2025 results as well as expectations for the future via conference call on Thursday, February 12, 2026 at 8:30 am ET. A live audio webcast of the call will be available on the Investors section of the Company’s website at
. An archived webcast will be available on the Alnylam website approximately two hours after the event.
About AMVUTTRA
®
(vutrisiran)
AMVUTTRA
®
(vutrisiran) is an RNAi therapeutic that delivers rapid knockdown of transthyretin (TTR), addressing the underlying cause of transthyretin (ATTR) amyloidosis. Administered quarterly via subcutaneous injection by a healthcare professional, AMVUTTRA is approved and marketed for the treatment of the polyneuropathy of hereditary transthyretin-mediated amyloidosis (hATTR-PN) in adults and for the treatment of the cardiomyopathy of wild-type or hereditary transthyretin-mediated amyloidosis (ATTR-CM) in adults to reduce cardiovascular mortality, cardiovascular hospitalizations and urgent heart failure visits. For more information about AMVUTTRA, including the full U.S.
Prescribing Information
, visit
AMVUTTRA.com
.
About ONPATTRO
®
(patisiran)
ONPATTRO is an RNAi therapeutic that is approved in the United States and Canada for the treatment of adults with hATTR amyloidosis with polyneuropathy. ONPATTRO is also approved in the European Union, Switzerland and Brazil for the treatment of hATTR amyloidosis in adults with Stage 1 or Stage 2 polyneuropathy, and in Japan for the treatment of hATTR amyloidosis with polyneuropathy. ONPATTRO is an intravenously administered RNAi therapeutic targeting transthyretin (TTR). It is designed to target and silence TTR messenger RNA, thereby reducing the production of TTR protein before it is made. Reducing the pathogenic protein leads to a reduction in amyloid deposits in tissues. For more information about ONPATTRO, including full
Prescribing Information
, visit
ONPATTRO.com
.
About GIVLAARI
®
(givosiran)
GIVLAARI (givosiran) is an RNAi therapeutic targeting aminolevulinic acid synthase 1 (ALAS1) approved in the United States and Brazil for the treatment of adults with acute hepatic porphyria (AHP). GIVLAARI is also approved in the European Union for the treatment of AHP in adults and adolescents aged 12 years and older. In the pivotal trial, GIVLAARI was shown to significantly reduce the rate of porphyria attacks that required hospitalizations, urgent healthcare visits or intravenous hemin administration at home compared to placebo. GIVLAARI is Alnylam’s first commercially available therapeutic based on its Enhanced Stabilization Chemistry ESC-GalNAc conjugate technology to increase potency and durability. GIVLAARI is administered via subcutaneous injection once monthly at a dose based on actual body weight and should be administered by a healthcare professional. GIVLAARI works by specifically reducing elevated levels of ALAS1 messenger RNA (mRNA), leading to reduction of toxins associated with attacks and other disease manifestations of AHP. For more information about GIVLAARI, including the full U.S.
Prescribing Information
, visit
GIVLAARI.com
.
About OXLUMO
®
(lumasiran)
OXLUMO (lumasiran) is an RNAi therapeutic targeting hydroxyacid oxidase 1 (HAO1). HAO1 encodes glycolate oxidase (GO). Thus, by silencing HAO1 and depleting the GO enzyme, OXLUMO inhibits production of oxalate – the metabolite that directly contributes to the pathophysiology of PH1. OXLUMO utilizes Alnylam’s Enhanced Stabilization Chemistry (ESC)-GalNAc-conjugate technology, which enables subcutaneous dosing with increased potency and durability and a wide therapeutic index.
Contacts
Alnylam Pharmaceuticals, Inc.
Christine Akinc
(Investors and Media)
617-682-4340
Josh Brodsky
(Investors)
617-551-8276
Read full story here
临床1期临床2期上市批准财报临床3期
2026-02-11
·今日头条
来源:市场资讯
(来源:财信证券研究)
小核酸药物优势明显,有望引领治疗范式升级。小核酸药物基于碱基互补配对原则作用于细胞内的pre-mRNA或mRNA,通过调控蛋白质的表达,从而实现治疗疾病的目的。相比于小分子和抗体药物,小核酸药物具有靶点丰富、特异性强、安全性更高、疗效更久、研发成功率高等优点,有望成为继小分子和抗体之后具有颠覆性的新主流疗法。
小核酸药物肝外递送技术突破,适应症延伸至慢病领域,商业化前景广阔。递送技术对小核酸药物的有效性和安全性起着关键作用。GalNAc递送技术为肝内递送的主流策略,已成功助力6款siRNA疗法获批上市,具有皮下注射给药、作用时效长达数月、高效靶向肝脏等优势,但存在组织局限性。借鉴GalNAc递送的技术思路,Avidity、Arrowhead、Alnylam等海外小核酸龙头企业分别开发出AOC 、TRiMTM、C16等肝外递送技术,成功实现对肺部、肌肉、中枢神经、脂肪等组织的靶向,极大拓宽了小核酸药物的作用范围。伴随着肝外递送技术突破,小核酸药物适应症从罕见病延伸至降血脂、降血压、减重等慢病领域,展现出广阔的发展前景。据瑞博生物港股招股说明书中的统计及预测数据, 2024-2029年,预计全球小核酸药物市场规模由57亿美元增长至206亿美元,期间复合增速约为29.40%。此外,小核酸药物交易活跃度提高,并诞生多笔重磅交易。例如,2025年10月,诺华以120亿美元收购AOC(抗体偶联寡核苷酸)领域的龙头企业Avidity Biosciences;2025年9月,诺华再次携手舶望制药,就多项心血管靶点siRNA资产展开合作,总交易金额超过53亿美元。
国内企业紧跟技术前沿,多项管线推进至临床II期。目前国内临床进展靠前的小核酸药物多为siRNA路线,多处于临床II期及以前阶段,从作用靶点来看,国内临床进展居前的小核酸药物靶点多为HBV、PCSK9、C3、ANGPTL3等。从适应症来看,国内临床进展居前的小核酸药物多集中在肝炎、心血管疾病、血脂异常、肾病等领域。从研发管线数量来看,舶望制药、瑞博生物的在研管线数量居前。
投资建议:维持医药生物行业“领先大市”评级,建议关注小核酸药物研发布局全面、产品创新程度高、研究进展靠前的药企,如恒瑞医药、悦康药业、前沿生物、信立泰等。
风险提示:产品研发及销售进展不及预期风险;行业竞争加剧风险;地缘政治风险;行业政策风险;医疗安全风险等。
1
行业简介
1.1 小核酸药物的发展历程、主要类型及作用机制
1998年,全球首款ASO药物福米韦生(Vitravene)获批,用于治疗巨细胞病毒视网膜炎,标志着小核酸疗法正式应用于临床。而早期的ASO疗法面临包括生物利用度差和脱靶效应在内的重大挑战,这限制了其临床应用。1998年,Andrew Zachary Fire和Craig Cameron Mello发现了RNAi机制(该发现于2006年获得诺贝尔奖),小核酸治疗迎来重大突破。2018年,全球首款siRNA药物Patisiran获批,标志着RNAi疗法正式落地。2019年,GalNAc偶联递送技术药物Givosiran上市,通过高效肝靶向性和缓释特性,系统性解决早期siRNA的稳定性和毒性问题。2020年,降胆固醇药物Inclisiran获欧盟批准,更将适应症从罕见病延伸至心血管慢性病领域,开辟全新市场空间。历经多年的技术发展,小核酸正迎来快速发展阶段,正成为从罕见遗传病到慢性病及癌症等多种疾病的有效治疗方式。截止目前,全球已有20余款小核酸药物获批上市。
小核酸药物,即寡核苷酸药物,是由十几个到几十个核苷酸串联组成的短链核酸,主要通过碱基互补配对原则作用于细胞内的pre-mRNA或mRNA,通过调控蛋白质的表达,从而实现治疗疾病的目的。广义的小核酸药物包括反义寡核苷酸(ASO)、小干扰核酸(siRNA)、微小RNA(miRNA)、核酸适配体(Aptamer)、小激活RNA(saRNA)、向导RNA(sgRNA)等。已获批和在研的核酸药物以ASO和siRNA、Aptamer为主。具体来看,siRNA是短双链RNA分子,当作为外源性治疗药物递送至细胞内时,可通过激活细胞内源性RNA干扰(RNAi)机制特异性降解靶标mRNA,已成为小核酸药物研发的前沿领域。ASO是单链RNA或DNA分子,可以和互补mRNA结合,通过多种机制调节蛋白质水平及功能。适配体则折叠形成特定的三维结构,以高亲和力和特异性结合靶标蛋白,从而阻断蛋白功能。
小核酸药物通过不同的机制发挥作用,调节基因表达和蛋白质水平,进而调控相应的蛋白质功能。小核酸药物的作用机制主要有以下几种:(1)基因沉默,如siRNA和miRNA可以通过与靶mRNA结合,形成RNA诱导的沉默复合物(RISC),对靶mRNA进行切割或抑制其翻译,从而实现基因沉默。(2)基因调控,ASO可以通过与靶mRNA结合,改变mRNA的剪接、稳定性或翻译效率,从而调节基因的表达。(3)免疫调节。一些小核酸药物可以通过激活或抑制免疫系统中的特定分子,调节免疫反应,发挥治疗作用。
siRNA药物的作用机制。siRNA疗法的生物学机制是荣获诺贝尔奖的RNAi机制,这是一种存在于包括人类和其他哺乳动物在内的广泛生物体中的天然基因调控机制。天然的RNAi过程涉及三个连续步骤:(1)前体RNA通过Dicer酶加工形成短双链RNA分子(20至25个核苷酸)(即微RNA),作为基因沉默的功能单元;(2)然后将这些微RNA分子装载到RNA诱导沉默复合体(RISC)中,在此实现双链解离,仅保留一条链作为引导链;(3)该引导链引导RISC结合靶mRNA,导致mRNA的翻译受到抑制并降解。前述过程称为基因沉默。通过独特的RNAi机制,siRNA疗法可通过精准设计沉默几乎任何基因,为解决过往不可成药靶点提供了重大机遇。此外,siRNA由于其基于序列的靶向机制,与其他模式相比,已经显示出多种优势,包括高特异性和(药效)长久持续,以及令人鼓舞的临床成功率。
反义寡核苷酸(ASO)的技术原理。ASO分子量较小(约18-30个核苷酸),是合成的单链核酸聚合物,可通过多种机制调节基因表达。ASO调控基因表达的途径有3种:(1)ASO与mRNA结合后,形成空间位阻,使mRNA不能再进入核糖体进行蛋白质翻译,使得这种mRNA所携带的基因信息表达下调。(2)ASO通过碱基互补配对,与靶标mRNA结合后,招募RNA酶将mRNA降解,同样使得基因表达下调。(3)主要是针对pre-mRNA在形成mRNA的过程中,ASO结合于Pre-mRNA的某个外显子区域,使得这段外显子被剪切掉,在最终生成的mRNA中不包含这段外显子。
1.2 小核酸药物优势:靶点丰富、特异性强、疗效持续时间长
相比于现有的小分子和抗体药物,小核酸药物具有靶点丰富、特异性强、安全性更高、疗效更久、研发成功率高等优点。作用靶点方面,根据弗若斯特沙利文的数据,在约20000种人类蛋白质中,传统小分子药物仅能靶向约15%的可成药蛋白。抗体药物虽在一定程度上扩展了靶向范围,但仍局限于细胞表面蛋白质,无法触及占人体蛋白质总量约80%的细胞内蛋白质。目前已获批疗法特异性靶向的人类蛋白质靶点总数不足700个。这使得绝大多数与疾病相关的蛋白质无法通过小分子药物和抗体疗法进行药物开发,即不可成药。与小分子和抗体药物相比,小核酸药物基于碱基互补配对原则以mRNA或其他RNA为靶点,作用于表达蛋白的上游过程,不受靶点成药性的限制,可选择的药物靶点更为丰富。在特异性及安全性方面,小核酸药物能够针对特定的基因序列进行干预,具有高度的特异性,这使得小核酸药物可以精准地治疗疾病,减少副作用。在疗效持续时间方面,通常来说,小分子药物的体内半衰期以小时计算,抗体药物的体内半衰期以周计算,而小核酸药物的体内半衰期以月计算,可显著降低给药频次,提高患者依从性。在研发成功率方面,小核酸药物的研发成功率更高,参考小核酸领域头部企业Alnylam的研发成功率,从临床I期到III期的累计转化率达到66.7%,相比靶向药(10.3%)和医药行业整体的研发成功率(5.7%)整整高6-12倍。伴随着技术的不断进步,小核酸药物有望成为继小分子和抗体之后具有颠覆性的新主流疗法。
2
行业发展
2.1 GalNAc为肝内递送主流策略,AOC等肝外递送技术崭露头角
小核酸药物的研发环节主要包括靶点筛选与序列设计、化学修饰、递送、合成及制剂等。其中,靶点筛选与序列设计主要是基于致病基因,设计并筛选出相对应RNA序列。小核酸药物的序列设计需同时考虑序列保守性、同源性、免疫原性、脱靶等因素,设计出合适的序列长度、核苷酸单体的含量、碱基单体的序列。化学修饰主要解决小核酸药物的不稳定、易被清除和降解、半衰期短等问题。ASO和siRNA当前主要是在磷酸骨架、核糖环、3'-和5'-末端进行化学修饰,提高其底物特异性和核酸酶抗性,并降低其毒性和免疫原性。递送系统主要是运载小核酸药物穿过细胞膜到达作用位点(胞浆或核内的mRNA),对小核酸药物的有效性和安全性起着关键作用。以siRNA为代表的小核酸具有分子量大(约14千道尔顿)、自身负电荷阻碍细胞膜穿透、容易被核酸酶降解等成药难点,需借助专门的递送系统克服前述挑战。小核酸药物递送方式主要有裸露RNA修饰递送、脂质体纳米递送(LNP)、GalNAc(N-乙酰半乳糖胺)递送等。
LNP是由功能性脂质、聚乙二醇修饰的脂质(PEGylated lipids)、饱和磷脂(DSPC)、和胆固醇构成的稳定纳米颗粒。功能性脂质为可电离的阳离子脂质体,在细胞渗透和释放中起到关键作用,是LNP成功开发的核心所在。聚乙二醇修饰的脂质的功能主要为:防止纳米颗粒在储存过程中和血液中的聚集;其含量可影响LNP粒径;延长体内摄取时间;可实现表面功能化,进而与配体或其他分子偶联。磷脂则有助于提供配方的整体结构稳定性,以及在生产和长期存储期间的稳定性。胆固醇因其疏水性,有助于提供刚性、配方稳定性并支持控释。LNP递送系统主要将小核酸药物包裹在脂质纳米颗粒(LNP)中,使被包裹的小核酸药物免于降解和清除,并促进其跨细胞膜运输到目标靶位。LNP通过肝细胞表面低密度脂蛋白受体识别和摄取,适用于治疗肝脏相关的疾病。2018年,Alnylam公司研发的基于脂质纳米颗粒(LNPs)递送的首款siRNA药物——Onpattro(Patisiran)获得美国FDA批准,用于治疗家族性淀粉样多发性神经病变。近年来的研究发现,通过调整构成LNP的脂质分子的类型和比例,能够诱导LNP被肝脏以外的器官或组织吸收。例如,基于ReCode Therapeutics公司的SORT-LNP平台开发的LNP载体已经进入临床开发阶段,用于将基于mRNA的疗法递送到肺部,治疗囊性纤维化等疾病;2024年9月,诺和诺德(Novo Nordisk)与NanoVation Therapeutics达成6亿美元的研发合作,基于后者的长循环LNP递送技术,将治疗药物递送到肝脏以外的组织,治疗心脏代谢疾病与罕见病。
GalNAc(N-乙酰半乳糖胺)递送技术于2014年被Alnylam实现,是小核酸药物发展历程中的重大突破,解决了小核酸药物靶向性差、脱靶效应严重、稳定性差的三大痛点。GalNAc递送系统由三个主体部分构成:特异性配体(通常是三个GalNAc糖分子)、连接臂(Linker,三价分支连接器)、治疗性寡核苷酸。GalNAc能特异性识别肝细胞表面高表达的去唾液酸糖蛋白受体(ASGPR),可实现高效肝脏靶向。GalNAc递送系统的作用机制为:GalNAc(N-乙酰半乳糖胺)通过与寡核苷酸共价连接,利用肝细胞上高度表达的去唾液酸糖蛋白受体(ASGPR),实现受体介导的细胞内吞。进入内体后的酸化环境促进受体–配体解离,从而释放寡核苷酸药物。ASGPR在内体酸化后快速回收至细胞表面,可反复参与配体结合,从而实现高效、可重复的肝摄取。相较于裸RNA、LNP递送系统,GalNAc递送系统的优势在于:可以通过皮下注射的方式达到较好的药物分布效果,并且作用时效长达数月;由于高效靶向肝脏,所需药物剂量小,副作用小。以ATTR领域上市的小核酸药物为例,相比仅经过化学修饰的Tegsedi(裸RNA)和通过LNP包裹的patisiran,采用GalNAc修饰递送的升级版药物Vutrisiran和Eplontersen在临床试验中表现出了良好的安全性和长效作用特点,vutrisiran在每三个月给药一次的频率下,降低血浆TTR效果类似于每3周给药一次30mg patisiran降低血浆TTR效果。依托于上述优势,GalNAc已成为肝内递送的主流策略。目前FDA批准的7款siRNA疗法,其中有6款采用了GalNAc偶联技术,首款GalNAc-siRNA药物Givosiran于2019年获FDA批准上市。GalNAc同样适用于ASO递送。2023年12月,首款GalNAc-ASO药物Eplontersen获FDA批准上市。由于ASGPR仅在肝实质细胞特异性高表达,GalNAc递送系统存在组织局限性,限制小核酸药物疾病领域的拓展。借鉴GalNAc偶联技术的思路,科学家们正在将与特定肝外组织结合的配体与寡核苷酸偶联,实现药物的靶向递送,AOC、TRiM、C16等肝外递送方式开始崭露头角。
AOC(抗体寡核苷酸偶联物)是将抗体与寡核苷酸通过连接子偶联后形成的复合物,其兼具了抗体的靶向性与寡核苷酸的治疗活性,结构形式上与ADC类似,只是有效载荷从小分子毒素替换成了具有基因调控功能的核酸药物。AOC的作用机制主要为:抗体负责识别并介导靶细胞的特异性内化,确保药物精准定位至目标组织;连接子维持体内稳定并在细胞内触发载荷进行释放,平衡药物循环稳定性与胞内起效效率;而寡核苷酸则通过与靶mRNA特异性互补结合,实现“基因沉默”或“剪接调控”,从根源上干预疾病进程。相较于以GalNAc递送为代表的传统寡核苷酸药物,AOC具备三大核心优势:一是肝外递送能力,通过选择不同的靶向抗体,可实现对肌肉、中枢神经、肿瘤等肝外组织的精准递送,例如针对TfR1靶点的AOC可穿透血脑屏障,为阿尔茨海默病、脊髓性肌萎缩症等中枢神经系统疾病提供新的治疗方案。二是更长的半衰期,抗体分子在血液中具有较强的稳定性,偶联后的AOC半衰期可达数天至数周,远长于传统寡核苷酸药物(通常为数小时至数天),大幅降低了给药频率。三是更低的脱靶毒性,由于AOC仅针对表达特定抗原的靶细胞发挥作用,对正常细胞的影响极小,有效减少了传统寡核苷酸药物可能引发的免疫反应、肝肾功能损伤等脱靶效应。Avidity是AOC领域的龙头公司,其AOC™技术平台专注于解决肌肉疾病治疗中的药物递送难题。Avidity旗下三款核心产品均迈入III期临床阶段(AOC 1020、AOC1044、AOC1001),分别用于治疗面肩肱肌营养不良病、杜氏肌营养不良症以及治疗强直肌营养不良。Avidity 的AOC™技术平台获得多家MCN的认可。2019年4月,礼来与Avidity达成一项全球许可和研究合作,借助Avidity的AOC平台开发免疫学和其他适应症药物,重点探索自身免疫疾病领域;2023年11月,BMS与Avidity达成潜在价值23亿美元的合作,利用Avidity的AOC平台开发最多5个抗体寡核苷酸偶联药物;2025年10月,诺华(Novartis)宣布以120亿美元收购Avidity,诺华将获得Avidity AOC™技术平台及三条后期研发管线。从AOC药物研发进展来看,海外企业处于临床后期阶段,国内企业多处于临床早期。
Arrowhead的TRiMTM递送技术平台主要由靶向配体、化学连接子、增强药代动力学的结构、小核酸四部分组成。该技术平台具有以下优势:(1)有效性,可产生深度且持续的效果,能够靶向以往成药难度大的靶点;(2)特异性,最大化活性和内在稳定性,降低潜在脱靶效应;(3)通用性,灵活的结构与设计支持多种给药途径及多组织靶向;(4)经济性,简洁的设计意味着相对较低的成本,并支持规模化生产。该技术平台可靶向肝脏、肺部、骨骼肌、中枢神经系统(CNS)、脂肪组织、眼部、心肌细胞,其中肝脏靶向已具备充分的临床验证,肺部靶向已经过深度的临床验证,骨骼肌、中枢神经系统(CNS)、脂肪组织靶向处于早期临床阶段。2025年12月10日,Arrowhead在第七届CNS递送峰会公布其中枢神经靶向的小核酸递送技术。该技术基于TRiMTM平台孵化,通过转铁蛋白受体(TfR1)靶向配体技术,突破BBB的递送瓶颈,可达大脑深部区域,并通过皮下注射实现便捷给药,具备每月至每季度给药的潜力。
Alnylam的C16偶联递送平台是一种利用脂质链-细胞膜相互作用的药物递送系统。十六烷基(C16)是一种连接到siRNA的短脂质链,其亲脂性得以与细胞膜相互作用,帮助药物穿透血脑屏障或肺部血管,实现精准递送。C16可以被多种细胞吸收,包括中枢神经系统和肺部的细胞。Alnylam基于C16平台已研发出3款处于临床阶段的siRNA疗法:ALN-APP(适应症为:脑淀粉样血管病、阿尔茨海默病)、ALN-HTT021(适应症为:亨廷顿病)、ALN-SOD(适应症为:肌萎缩侧索硬化症)。
2.2 小核酸药物适应症从罕见病延伸至心血管、减重等慢性病领域
从已获批上市的产品来看,小核酸在罕见病适应症上率先获得突破,包括杜氏肌营养不良、脊髓性肌肉萎缩症、转甲状腺素蛋白淀粉样变性、血友病、家族性高乳糜微粒血症等。从在研管线的适应症来看,排名前五的疾病依次是肿瘤(24%)、遗传病(22%)、感觉器官疾病(13%)、心血管系统疾病(12%)、消化代谢性疾病(9%)。此外,小核酸药物也在皮肤病、血液病、泌尿、感染等多个领域布局。伴随着小核酸药物技术的进步,小核酸药物在慢病治疗领域的应用正逐渐从罕见病向高血脂、高血压、减重等常见慢病延伸。2020年12月,降胆固醇药物Inclisiran获欧盟批准,小核酸药物适应症从罕见病延伸至心血管慢性病领域。
2.2.1 高血脂:靶向APOC3、PCSK9的小核酸已获批,Lp(a)靶点的II期数据积极
血脂异常是一种以血液中任何或所有脂质(如甘油三酯、胆固醇、磷脂)或脂蛋白水平异常为特征的疾病。在全球范围内,成人血脂异常的患病率估计约为40%,每年影响约30亿人。高胆固醇血症(HC)是最常见的一种血脂异常,约占全球血脂异常病例的27.4%,典型特征是LDL-C(低密度脂蛋白胆固醇)升高,伴或不伴总胆固醇升高。高甘油三酯血症(HTG)定义为血液甘油三酯水平升高(TG≥1.7 mmol/L),影响全球约25%的血脂异常病例。长期高脂血症可能导致动脉粥样硬化,增加心脑血管疾病的风险。无论是高胆固醇血症(HC)还是高甘油三酯血症(HTG),当前标准治疗均存在疗效局限、依从性差和安全性风险等问题,尤其是对重度或难治性病例,临床迫切需要更高效、长效且安全的新型治疗选择。肝脏是脂代谢的重要路径,转运内源性甘油三酯和胆固醇的脂蛋白在肝脏合作,刺激肝脏脂蛋白合成因子会导致血脂升高。因此,擅长肝靶向的小核酸药物在降血脂方面展现出良好的治疗前景。近年新兴降脂治疗靶点不断涌现,包括载脂蛋白C(ApoC)、脂蛋白a(Lp(a))、血管生成素样蛋白(ANGPTL)、前蛋白转化酶枯草溶菌素9(PCSK9)等。
载脂蛋白C3(APOC3)是体内脂质代谢的关键调节因子,尤其影响甘油三酯(TG)水平。当APOC3水平升高时,APOC3主要通过以下两种途径破坏正常的脂质水解和清除:一是抑制负责分解TG的酶——脂蛋白脂肪酶(LPL)(LPL依赖性途径);二是干扰肝脏对富含TG的残余脂蛋白的清除(LPL非依赖性途径)。靶向APOC3的小干扰RNA(siRNA)疗法通过特异性靶向APOC3 mRNA,有效降低APOC3蛋白水平,从而消除其对脂蛋白脂肪酶等关键脂质代谢酶的抑制作用,并提高富含TG的脂蛋白的清除率。截止目前,全球已有两款靶向APOC3的反义寡核苷酸(ASO)药物(volanesorsen和olezarsen)获批上市,用于治疗家族性乳糜微粒血症综合征(FCS)——一种由乳糜微粒代谢缺陷引起的罕见、遗传性严重高甘油三酯血症(HTG)。2025年11月18日,美国FDA批准了Arrowhead公司的RNAi疗法普乐司兰钠注射液(Plozasiran)的新药上市申请。Plozasiran可作为饮食疗法的辅助手段,用于降低家族性乳糜微粒血症(FCS)成人患者的甘油三酯水平。Plozasiran是目前唯一一个获得FDA批准用于治疗FCS的siRNA药物,患者可在家中自行给药,只需每三个月进行一次简单的皮下注射即可。III期临床试验PALISADE的结果显示,25mg Redemplo实现了显著且持久的甘油三酯降低,中位数相较基线变化为-80%,而安慰剂数据合并组为-17%。除FCS适应症外,Plozasiran也正在进行针对严重高甘油三酯血症、混合性高脂血症的临床研究。Volanesorsen、olezarsen、Plozasiran验证了APOC3作为治疗靶点在重症HTG中的临床有效性。
PCSK9通过促进肝细胞表面的低密度脂蛋白(LDL,即“坏胆固醇”的主要载体)受体降解,从而自然调节胆固醇代谢。PCSK9抑制剂通过阻止这一降解过程发挥作用,从而增加功能性LDL受体的数量,并增强肝脏对LDL-C(低密度脂蛋白胆固醇)的清除能力。临床试验证实,该机制能够实现显著的LDL-C降低效果(通常可达50%至70%),而相比之下,他汀类药物的降幅一般为20%至50%,依折麦布约为15%至20%。PCSK9抑制剂对于他汀类药物不耐受,或通过常规疗法难以达到血脂目标的高危患者尤为有效。靶向PCSK9的siRNA药物通过在mRNA水平上抑制PCSK9蛋白的合成。与主要作用于循环中PCSK9蛋白的单克隆抗体不同,siRNA药物能够从源头上阻断肝细胞内PCSK9的生成,从而持续增加LDL受体的表达,并持久提升LDL-C的清除效率。针对PCSK9的siRNA药物的临床试验表明,其疗效持久,每年仅需给药两次即可实现长期的LDL-C控制,而抗体药物通常需要每两周或每月注射一次。目前,全球已有一款靶向PCSK9的siRNA药物——英克司兰(Inclisiran)获批用于治疗高胆固醇血症(HC)。此外,全球范围内另有多款用于治疗HC的siRNA候选药物正处于临床开发阶段。
大分子脂蛋白Lp(a)由低密度脂蛋白样颗粒和载脂蛋白a[Apo(a)]共价结合而成。Lp(a)水平的增加与心血管疾病风险的增加直接相关。一项发表在杂志Circulation上的荟萃分析研究结果表明,Lp(a)水平与ASCVD(动脉粥样硬化性心血管疾病)风险存在相关性,且与基线LDL-C(低密度脂蛋白胆固醇)水平无关,即Lp(a)与LDL-C对ASCVD风险的影响是独立叠加的,LDL-C水平的降低并不能完全抵消Lp(a)介导的ASCVD风险。《中国血脂管理指南(2023)》已明确指出,Lp(a)升高是冠心病、缺血性脑卒中、外周血管疾病、冠状动脉钙化等心血管疾病的独立危险因素。由于Lp(a)主要在小核酸药物的优势作用器官——肝脏中合成,多家药企已布局靶向Lp(a)的小核酸降脂药物研发。靶向Lp(a)的小核酸降脂药物的主要作用机制为:通过阻止核糖体在mRNA上的移动和翻译,使目标mRNA被切割为不具有翻译功能的片段,通过影响基因的表达从源头上阻止Lp(a)的形成。
目前已有多款靶向Lp(a)的小核酸降脂药物在临床中取得显著效果,并进入到Ⅲ期阶段。Pelacarsen(诺华和Ionis联合研发,处于临床III期)公布的临床Ⅱ期研究(NCT03070782)结果显示,Pelacarsen降低Lp(a)水平的作用呈剂量依赖性,且5个试验组Lp(a)水平降低35%~80%,其中80mg/月的给药剂量可使98%的患者Lp(a)水平降至50mg/dL以下。Olpasiran(安进2016年从Arrowhead引进,处于临床III期)的Ⅱ期研究(NCT04270760)结果显示,每12周接受75mg剂量Olpasiran的患者,在治疗36周时血液中Lp(a)水平下降为97.4%;Olpasiran各剂量组患者的LDL-C和载脂蛋白B水平也显著降低;超过75mg剂量的参与者在末次给药后近一年内,Lp(a)水平仍显著降低约40%~50%,Olpasiran展现出持久的降脂能力。Zerlasiran(Silence Therapeutics开发)的Ⅱ期临床(NCT05537571)数据显示,36周治疗后,Zerlasiran各治疗组均可显著降低Lp(a)水平。其中,接受450mg每24周两次治疗的患者,Lp(a)平均水平降低85.6%;接受300mg每16周三次治疗的患者,Lp(a)平均水平降低82.8%,接受300mg每24周两次治疗的患者,Lp(a)水平降低81.3%。
2.2.2 高血压:显著提升患者依从性,zilebesiran已达到II期临床终点
主流的高血压药物降压效果均较为明显,但需每日服用,患者依从性较差,显著影响了高血压治疗率的提升。小核酸药物有望借助疗效更佳持久的优势,解决高血压药物依从性差的短板。目前,全球处于活跃状态的针对高血压适应症的小核酸疗法超过10款,其中该疗法先驱是Ionis的IONIS-AGT-LRx(目前已不再活跃),目前研究进展较快的管线有:Alnylam的Zilebesiran(siRNA)、Ionis的Tonlamarsen(ASO)、诺华的QCZ-484(siRNA)。国内进展相对靠前的高血压小核酸疗法主要有:先衍生物的LDR-2402、恒瑞医药的HRS-9563等。从作用靶点来看,在研的高血压在研疗法主要集中在AGT靶点。AGT作为肾素-血管紧张素-醛固酮系统的更上游的靶点,在血压调节中的作用更为关键,虽然其在肝脏、肾脏、心脏和血管内皮细胞等众多器官组织中表达,但血液循环中的AGT主要来自肝脏的表达,而肝脏正好是小核酸疗法的优势器官。此外,小核酸药物虽然抑制了循环系统整体的RAAS活性,导致血压降低,但不会抑制肾脏局部的RAAS活性,不会导致肾脏的血流灌注过低。小核酸技术与AGT靶点完美契合,导致AGT靶向降压药均选择小核酸技术。
2024年4月,Alnylam和罗氏共同宣布,RNAi疗法zilebesiran治疗高血压的II期KARDIA-2研究达到主要终点。数据显示,将zilebesiran添加到标准治疗方案中,能在第3个月显著降低患者的24小时平均收缩压(SBP),最高可将SBP额外降低12.1mmHg,几乎媲美ARNI+CCB的临床有效性,且部分接受一针zilebesiran的患者在6个月后随访时,血压持续降低的效果仍能维持。安全性方面,实验中最常见不良事件为轻度高钾血症(5.5% vs 1.8%)、低血压(4.3% vs 2.1%)和急性肾功能波动(4.9% vs 1.5%),多数无需干预即可缓解,严重不良事件发生率与安慰剂组无差异。小核酸疗法本身的局限性也导致zilebesiran的起效速度相对缓慢,通常情况下给药后数周后才能达到降压效果,因此不适合于高血压急症的单一治疗手段。未来最佳应用场景应当是与一线疗法联合。
2.2.3 减重:Arrowhead与Wave等药企布局领先,临床前及临床I期数据积极
当前GLP-1类减重药物存在停药后体重反弹率高、肌肉流失、给药周期短等短板。依托全新的作用机制,以RNAi为核心的小核酸药物有望解决GLP-1类减重药物的短板。目前,全球共有20余款小核酸减重管线,其中6款已进入I期及以上临床阶段,另有14款处于临床前研究阶段。在研管线中,INHBE、ALK7是多数药企选择的靶点。INHBE和ALK7同属TGFβ超家族,构成肝脏与脂肪组织间的代谢调控轴。INHBE在肝脏中表达,其产物激活素E(Activin E)分泌入血后,结合脂肪细胞表面的ALK7受体,激活Smad2/3信号通路,进而抑制脂肪分解、促进脂质储存并诱导胰岛素抵抗。ALK7(活化素受体样激酶7)主要在脂肪组织中表达,是INHBE的作用受体,抑制ALK7信号通路,可以直接促进脂肪分解,改善胰岛素抵抗。从小核酸减重药物研发进展来看,Arrowhead和Wave等在小核酸减重领域的进展相对靠前,Arrowhead的ARO-INHBE(靶向INHBE)和ARO-ALK7(靶向ALK7)处于I/IIa期阶段,Wave的WVE-007(靶向INHBE)读出积极的I期中期数据。此外,Alnylam在小核酸减重领域的管线进展稍慢,但布局较为全面, IND-enabling阶段的分子覆盖了INHBE(肝脏靶向)、ALK7(脂肪靶向)、Gene D(肌肉靶向)等靶点。
Arrowhead的ARO-INHBE旨在降低INHBE基因及其分泌的基因产物Activin E在肝脏的表达。INHBE基因编码的是抑制素亚基βE(Inhibin Subunit Beta E),是转化生长因子-β(TGF-β)蛋白质超家族的成员,主要在肝脏中表达;其肝脏表达水平与人类的胰岛素抵抗和体重指数正相关。ARO-INHBE的临床前研究表明:(1)食蟹猴模型显示,ARO-INHBE在D1和D29以3mpk的皮下注射实现了INHBE转录物的深度敲低,持续时间至少为85天。(2)在DIO和db/db小鼠模型中,ARO-INHBE可抑制小鼠体重增加19%。ARO-INHBE于2024年12月进入I/IIa期临床研究,预计2026年初公布I/IIa期临床研究的初始数据。
Arrowhead的ARO-ALK7为全球首款靶向脂肪组织的RNAi在研疗法,旨在降低Activin受体样激酶 7 (ALK7) 在脂肪中的表达。ARO-ALK7的临床前研究数据显示:(1)在DIO小鼠模型中,每周注射3 mg/kg ARO-ALK7,第16周时,腹股沟白色脂肪组织和性腺周围白色脂肪组织中ALK7 mRNA表达量分别下调80%、40%;相比于生理盐水对照组,ARO-ALK7可显著抑制体重增加39%,脂肪量减少约50%,并保持瘦肉体重。(2)在食蟹猴模型中,单次注射3mg/kg剂量的ARO-ALK7,1个月后脂肪组织中的ALK7 mRNA沉默89%,并在12周内保持75%左右的沉默水平。ARO-ALK7于2025年5月进入I/IIa期临床研究。
2025年12月,Wave Life Sciences公布了其肥胖症候选药物WVE-007的I期INLIGHT试验中期数据。WVE-007是一种基于GalNAc-siRNA技术的RNA干扰药物,通过Wave proprietary的SpiNA设计,精准沉默肝脏中的INHBE基因mRNA,从而降低其下游蛋白产物Activin E的表达水平。与传统GLP-1受体激动剂通过中枢食欲抑制实现减重不同,WVE-007通过调控脂肪代谢和肌肉保护的相关通路,在减少脂肪组织的同时促进瘦体重维持甚至增长。WVE-007的I期INLIGHT试验结果显示,单次240mg皮下注射后12周,32名BMI 28-35 kg/m²的受试者内脏脂肪减少9.4%(p=0.02),全身脂肪降低4.5%,瘦体重增加3.2%(约4磅),总体重因肌肉增长抵消仅下降0.9%。血清Activin E水平在43天时达到最大降幅78%,疗效维持超过85天,支持每年1-2次给药的潜力。安全性方面,在已评估的240mg至600mg剂量范围内,所有治疗相关不良事件均为轻度,未报告中度或重度事件,无严重不良事件或治疗中断病例。试验未观察到GLP-1类药物常见的胃肠道副作用(恶心、呕吐、腹泻),且血脂与肝功能检测未见临床意义变化。Wave计划在2026年Q1发布240mg队列6个月数据和400mg队列3个月数据,并启动II期试验探索在更高BMI及合并症患者中的应用。
2.3 小核酸药物交易活跃度提高,多家国内企业与MCN达成交易
伴随着小核酸技术突破以及相关药物成功上市等,小核酸药物交易活跃度呈现提升趋势,具体表现为:小核酸药物的交易金额、首付款逐年增加,小核酸license-out项目占比不断提高,诞生多笔重磅交易。从重磅交易来看,2019年11月,诺华以97亿美元收购TMC,获得靶向PCSK9的长效siRNA降脂药Inclisiran(Leqvio)。2025年10月,诺华以120亿美元收购AOC(抗体偶联寡核苷酸)领域的龙头企业Avidity Biosciences,获得Avidity的神经学管线和差异化技术平台。从国内企业授权交易来看,舶望制药、圣因生物等企业先后与礼来、诺华等国际大药企达成授权交易,授权合作内容包括在研项目以及技术平台等。2024年1月,诺华以41.65亿美元的总交易金额获得舶望制药多款心血管siRNA药物的海外权益。2025年9月,诺华再次携手舶望制药,就多项心血管靶点siRNA资产展开合作,总交易金额超过53亿美元。2025年11月,礼来与圣因生物达成一项价值最高达12亿美元的合作协议,双方将基于圣因生物专有的LEAD™平台共同推动针对代谢性疾病的RNAi候选药物的开发。LEAD™平台是圣因生物自主研发的一种组织选择性递送技术,能够实现RNAi药物在肝外组织与细胞中的高效、特异性递送。
2.4 商业化前景广阔,部分已上市产品销售表现亮眼
依托于独特的治疗优势、技术突破、适应症扩展以及上市产品增加,全球小核酸药物市场将呈现快速增长。据瑞博生物港股招股说明书中的统计及预测数据,2019-2024年,全球小核酸药物市场规模由27亿美元增长至57亿美元,期间复合增速约为16.20%。2024-2029年,预计全球小核酸药物市场规模由57亿美元增长至206亿美元,期间复合增速约为29.40%;2029-2034年,预计全球小核酸药物市场规模由206亿美元增长至549亿美元,期间复合增速约为21.60%。单独看siRNA药物,据靖因药业港股招股说明书中的统计及预测数据,2024-2029年,预计全球siRNA药物市场规模由24亿美元增长至100亿美元,期间复合增速约为33.03%;2029-2034年,预计全球siRNA药物市场规模由100亿美元增长至294亿美元,期间复合增速约为24.07%。
部分已上市小核酸药物销售表现亮眼。诺华Leqvio(靶向PCSK9 mRNA的siRNA疗法)于2020年12月在欧盟获批上市,用于治疗成人高胆固醇血症或混合性血脂异常,并于2021年12月获得美国FDA批准,作为饮食和他汀类药物治疗的辅助药物,用于治疗患有原发性高脂血症的成年患者。2025年8月,美国FDA批准了Leqvio的扩展适应症申请,允许其作为单药,与饮食控制和运动联合使用,以降低成人高胆固醇血症患者的低密度脂蛋白胆固醇(LDL-C)水平。自获批上市以来,Leqvio销售额呈现快速增长趋势。2024年,诺华Leqvio实现销售收入7.54亿美元,同比增长超100%。2025年前三季度,Leqvio实现销售收入8.63亿美元,同比增长61%。诺华预计Leqvio销售额峰值有望达到40亿美元。此外,小核酸药物龙头企业Alnylam营收的快速增长也反映出小核酸药物具备广阔的商业化前景。2020-2024年,受益于siRNA产品上市放量,Alnylam的营业收入由4.93亿美元增长至22.48亿美元。2025年前三季度,Alnylam实现营业收入26.17亿美元,其中产品净收入19.92亿美元,同比增长67%。具体来看,TTR产品线表现亮眼,实现收入16.28亿美元,同比增长85%,主要由Amvuttra(14.87亿美元)和Onpattro(1.41亿美元)贡献。受益于转甲状腺素蛋白淀粉样变性心肌病(ATTR-CM)适应症获批,Amvuttra的2025年第三季度收入同比增长165%至6.85亿美元。
3
相关企业
3.1 国外:管线进展领先,多组织靶向可期
(1)Alnylam:全球RNAi疗法先驱及领导者,致力于2030年前实现主要组织递送
Alnylam是RNAi治疗领域的开创者,成立于2002年,于2018年实现公司首个RNAi药物(也为全球首款)商业化。截止目前,公司已有6款RNAi产品获批上市,7项管线处于临床III期阶段,17项管线处于临床II或I期阶段。公司产品管线聚焦的疾病领域包括:TTR(转甲状腺素蛋白淀粉样变性疾病)、罕见病、心血管、代谢、神经系统疾病。其中,在TTR疾病领域,Alnylam进行深入布局,并不断升级迭代TTR靶向药物,具体产品管线包括:(1)静脉注射、每三周给药的Patisiran;(2)皮下注射、每三个月给药的Vutrisiran;(3)疗效更好、每半年给药、处于临床III期的Nucresiran。受益于Vutrisiran(Amvuttra)ATTR-CM成人患者适应症获批,TTR板块收入呈现快速增长,已成为Alnylam营收增长的主要动力。
Alnylam开发了LNP、GalNAc、C16偶联等多种RNAi递送技术。LNP(脂质纳米颗粒)递送技术:主要靶向肝脏,基于LNP技术的RNAi疗法通过静脉注射给药。Alnylam首个获批的RNAi疗法药物ONPATTRO®(patisiran)基于LNP递送技术,该技术也用于开发COVID-19疫苗。GalNAc(N-乙酰半乳糖胺)偶联物递送技术:主要靶向肝脏,基于GalNAc偶联技术的RNAi疗法通过皮下注射给药。Alnylam已获批的GIVLAARI®、OXLUMO®、AMVUTTRA®以及Leqvio®均采用GalNAc偶联物递送技术。C16偶联递送技术:可靶向中枢神经系统、肺部和眼部细胞等,基于C16偶联技术的RNAi疗法通过鞘内注射(经脊髓注射)给药。Alnylam在研RNAi疗法ALN-APP(针对淀粉样前体蛋白,用于治疗阿尔茨海默病和脑淀粉样血管病)采用了C16偶联技术。在2025 R&D Day上,Alnylam宣布递送远景:在2030年前,实现所有主要组织布局。
(2)Arrowhead:TRiM™技术可靶向多组织,多项管线授权MCN
Arrowhead基于TRiM™平台,成功开发了靶向肝脏、肺、肌肉、脂肪、中枢神经的多种RNAi疗法,包括4项处于临床III期的管线:①Plozasiran。2025年11月,美国FDA批准Arrowhead公司的RNAi疗法Plozasiran(也称普乐司兰钠注射液或Redemplo,靶向APOC3基因)的新药上市申请,Plozasiran可作为饮食疗法的辅助手段,用于降低家族性乳糜微粒血症(FCS)成人患者的甘油三酯水平。Plozasiran治疗重度高甘油三酯血症的III期临床试验预计2026Q3公布结果。②用于治疗纯合子家族性高胆固醇血症(HoFH)的ANGPTL3靶向RNAi疗法Zodasiran。③Arrowhead和武田(Takeda)合作开发的、用于治疗α1-抗胰蛋白酶缺乏症相关的肝病(AATD-LD)的Fazirsiran。II期结果显示,fazirsiran对肝病关键标志物具有良好的影响。④Arrowhead和安进合作开发、用于治疗动脉粥样硬化性心血管疾病(ASCVD)、Lp(a)基因靶向的siRNA疗法olpasiran,预计在2026年年底公布初步结果。olpasiran的II期临床试验结果显示,12周给药一次,高剂量组在治疗36周时Lp(a)降低超90%,效果持续至48周。
Arrowhead与诺华、赛诺菲、GSK、武田、安井等国际大药企达成多项研发合作。2025年9月,诺华与Arrowhead达成潜在交易总额20亿美元的合作协议,共同开发处于临床前的用于治疗帕金森病的药物ARO-SNCA。2025年8月,赛诺菲与Arrowhead的子公司维亚臻(Visirna Therapeutics)签署资产购买协议,赛诺菲以1.3亿美元预付款、最高达2.65亿美元里程碑付款的价格获得在大中华区开发和商业化在研药物普乐司兰钠注射液(Plozasiran)的权利。普乐司兰钠注射液用于治疗FCS(家族性乳糜微粒血症综合征)的新药上市申请于2025年1月26日获得NMPA的正式受理,且已获得中国NMPA的突破性疗法认定和优先审评资格。2021年11月,Arrowhead与葛兰素史克(GSK)达成RNAi疗法ARO-HSD(用于治疗非酒精性脂肪性肝炎-NASH)的研发合作协,Arrowhead将获得1.2亿美元前期付款,并有资格获得超过10亿美元的开发和商业化里程碑付款。2020年10月,武田(Takeda)和Arrowhead达成RNAi疗法ARO-AAT(用于治疗α-1抗胰蛋白酶相关肝病-AATLD)的研发合作协议,Arrowhead将获得3亿美元的前期付款,并有资格获得高达7.4亿美元的潜在开发、监管和商业化里程碑付款。2016年9月,安进以5650万美元预付款以及最高可达6.17亿美元的额外付款获得Arrowhead的Olpasiran(用于高血脂患者的心血管事件二级预防)的全球独家选择权。
3.2 国内:紧跟技术前沿,多项管线推进至临床II期
目前国内临床进展靠前的小核酸药物多为siRNA路线,多处于临床II期及以前阶段,其中维亚臻生物的VSA003已经推进到临床Ⅲ期阶段。从作用靶点来看,国内临床进展居前的小核酸药物靶点多为HBV、PCSK9、C3、ANGPTL3等。从适应症来看,国内临床进展居前的小核酸药物多集中在肝炎、心血管疾病、血脂异常、肾病等领域。从研发管线数量来看,舶望制药、瑞博生物的在研管线数量居前。
(1)瑞博生物:小核酸药物开发的先驱及领导者,多项管线处于临床II期
技术平台:公司依托国际化研发平台,实现小核酸药物全周期技术覆盖,包括药物递送、化学修饰、多靶点药物研发、模型引导的药物开发及药学开发生产。其中,RiboGalSTARTM是肝组织靶向递送技术,已推动了六个项目进入临床开发,其高效、长效及良好的安全性已在临床中得到验证。RiboGalSTARTM已成为全球最成功的GalNAc递送技术之一,并已在包括中国、欧洲和美国在内的主要司法管辖区获得专利授权。2024年1月,瑞博生物与德国勃林格殷格翰达成合作,双方将基于RIBO-GalSTARTM技术平台共同开发治疗非酒精性或代谢功能障碍相关脂肪性肝炎(NASH/MASH)的小核酸创新疗法,该项合作总交易金额超过20亿美元。RiboOncoSTARTM是一项领先的肿瘤靶向平台,采用核酸缀合递送技术,支持多个潜在“同类首创”癌症治疗项目的开发。在临床前研究中,RiboOncoSTARTM在特定癌症(包括胶质瘤、胰腺癌)中表现出优于标准治疗的抗肿瘤效果和安全性。RiboPepSTARTM通过靶向分子缀合实现对多种肝外器官的靶向递送,在肾脏和中枢神经系统(CNS)相关的多个临床前疾病模型中,该平台展现出优于现有疗法的显著疗效,并具有良好的安全性。
瑞博生物的研发管线覆盖心血管、代谢和肾脏疾病、肝病及其他治疗领域。在心血管、代谢和肾脏疾病领域,RBD4059(血栓性疾病)、RBD5044(高甘油三酯血症)、RBD7022(高胆固醇血症)已处于全球临床II期阶段。其中,RBD4059是全球首款、临床开发进度最快、针对FXI靶点的siRNA药物(First-In-Modality);RBD5044是全球第二款进入临床开发的靶向ApoC3的siRNA。在肝病领域,RBD1016(乙型肝炎、丙型肝炎)处于临床II期阶段。在II期临床研究中,RBD1016表现出良好的耐受性安全性,并对乙肝表面抗原(HBsAg)显示出强大的可持续至6个月的抑制作用。RBD1016有望作为未来联合治疗方案中达到慢性乙型肝炎(CHB)功能性治愈的基石疗法,以及丙型肝炎(CHD)的领先siRNA候选药物。
(2)舶望制药:siRNA疗法管线布局广泛,持续获得国际大药企认可
舶望制药核心管理层具备丰富的小核酸药物研发经验,两位联合创始人曾在Arrowhead Pharmaceuticals担任要职。舒东旭博士(联合创始人、首席执行官)曾任Arrowhead Pharmaceuticals高级科学家,专注于肝外递送平台的开发,其中部分成果已成功应用于临床研究。邵鹏程博士(联合创始人、首席科学官)曾在默克研究实验室参与多个心血管健康、糖尿病、肿瘤学和中枢神经系统疾病等项目;曾担任Arrowhead Pharmaceuticals药物化学总监,领导肝外递送平台和项目工作;曾领导专门从事基于siRNA的NASH治疗新药研发的初创公司Mitotherapeutix的研发工作。金建军博士(首席医学官)曾在惠氏、勃林格殷格翰和礼来中国担任临床开发和医疗事务治疗领域负责人;曾担任诺华中国临床开发负责人,成功带领团队进行了siRNA药物LEQVIO®(inclisiran)的亚洲3期研究。
舶望制药siRNA疗法管线布局广泛,涉及心血管、罕见病、中枢神经系统、病毒性肝炎、代谢性疾病等多个重要治疗领域,并与国际大药企达成多项授权合作。其中,BW-01(心血管疾病)、BW-02(心血管疾病)、PKK(遗传性血管水肿)处于临床II期阶段。舶望制药在肥胖、代谢功能障碍脂肪肝炎、阿尔茨海默症、帕金森病等领域开发了多个处于早期发现阶段的项目。此外,舶望制药的在研管线质量持续获得国际大药企认可。2024年1月,诺华以41.65亿美元的总交易金额获得舶望制药多款心血管siRNA药物的海外权益。2025年9月,诺华再次携手舶望制药,就多项心血管靶点siRNA资产展开合作,总交易金额超过53亿美元。
(3)悦康药业:ASO药物CT102完成Ⅱa期临床,搭建GalNAc、TLP递送平台
专利布局:在核酸药物递送领域,包括LNP、TLP、GalNAc递送技术在内,截至2025年6月30日,公司已获得了25项发明专利授权。其中,小核酸肝靶向递送技术GalNAc已成功获得5项发明专利授权,其PCT专利已进入美国、欧洲等地区,专利中的GalNAc采用独特的设计,具有全新化学结构,属于新一代GalNAc技术。可电离阳离子脂质辅料YK-009先后获得中国(含港澳台)、美国、日本、以色列、马来西亚、南非的发明专利授权,成功应用于公司多款mRNA疫苗管线。具有全新结构的Cap1型加帽类似物,成功获得两项发明专利授权,攻克了mRNA疫苗和药物相关领域发展的“卡脖子”的难题。
技术平台:公司已搭建了以核酸药物为基础的领先的靶点发现平台、高通量筛选平台、领先的工艺开发及规模化制备平台、完整的分析质控平台,并在AI靶点发现、序列优化设计、LNP递送、GalNAc递送、TLP递送、肝外靶向递送、核苷单体修饰、共加帽等核酸药物底层技术上进行了系统深度布局。其中,公司自主研发的肿瘤免疫治疗递送系统——三组分脂质组合物TLP,相比于现有mRNA代表性的LNP递送技术,TLP展现出了显著优势。
在研管线:公司已有多款siRNA药物和mRNA疫苗进入临床研究阶段,适应症覆盖原发性肝癌、高血脂、高血压、乙型肝炎、带状疱疹、呼吸道合胞病毒(RSV)、实体肿瘤等疾病领域。其中,治疗原发性肝癌的国内首款反义核酸药物——注射用CT102的IIa期临床试验已完成;YKYY015注射液是目前国内处在临床阶段的同类PCSK9 siRNA项目中唯一在美国IND获批的全球潜在Best-in-class超长效siRNA降脂药物;YKYY029注射液是具有全新结构的靶向AGT基因的小干扰核糖核酸(siRNA)药物,已在中国、美国获批开展临床试验;YKYY032注射液是靶向Lp(a)蛋白的双链siRNA药物,已在中国、美国获批开展临床试验。
(4)其他企业
恒瑞医药:siRNA疗法HRS-5635(靶向HBV)治疗慢性乙肝适应症处于II期临床阶段;siRNA疗法HRS-9563治疗轻至中度高血压的II临床试验方案已于2025年12月16日公示。
信立泰:2025年5月,信立泰与成都国为生物达成合作协议,获得国为生物在研AGT-siRNA药物GW906在大中华区的独家许可权益。GW906为肝靶向配体偶联siRNA药物,通过特异性沉默肝脏血管紧张素原(AGT)mRNA,抑制肾素-血管紧张素-醛固酮系统(RAAS)最上游前体蛋白的合成,从而阻断血管紧张素II的生成路径,实现长效降压效应。GW906以原发性高血压为靶向适应症,目前正处于临床I期研究阶段。此外,信立泰也积极布局INHBE靶向、用于治疗心血管与代谢疾病的siRNA疗法,目前处于临床前研究阶段。
迈威生物:2025年9月17日,迈威生物与AditumBio宣布成立KalexoBio,并就心血管领域双靶点siRNA创新药2MW7141达成全球独家授权协议。根据协议,迈威生物将获得最高可达10亿美元的预付款与里程碑付款,以及低个位数的特许权使用费,其中包括1200万美元的首付款和近端付款。2MW7141是一款处于临床前阶段的双靶点小核酸药物,主要针对血脂异常人群的血脂调控以及高危心血管事件的预防。2MW7141协同调控效应明确,其在临床前研究中展现出对靶基因强效且持久的抑制效果,并且脱靶风险较低,有望成为首款采用非LNP递送系统的双靶点siRNA药物。
前沿生物:在研siRNA药物已覆盖IgA肾病、血脂异常、内分泌相关、痛风、肌肉、中枢神经等疾病领域,以及肿瘤治疗领域。在IgA肾病领域,公司布局了三款靶向补体系统的小核酸药物:FB7013、FB7011、FB7014。其中,FB7013(单靶点小核酸药物)已建立放大工艺并完成GMP批次生产,预计2025年底递交IND申请。FB7011(双靶点小核酸药物)处于临床前开发阶段,临床前研究表明,FB7011显示出具有更高疗效、更好安全性的潜在优势。FB7014(单靶点小核酸药物)处于临床前开发阶段,临床前药效研究数据显示,FB7014具有强效持久的疗效,且安全性良好。在血脂异常异常领域,公司布局了针对不同脂蛋白靶点的小核酸药物,包括FB7023和FB7022(靶向ANGPTL3),目前均处于临床前研究阶段。在内分泌领域,公司在代谢相关脂肪性肝炎(MASH))、2型糖尿病(T2DM)等方面布局了多款具有自主知识产权的小核酸药物,已提交4项发明专利申请,相关项目均处于临床前研究阶段。此外,公司依托内部成熟的化学合成偶联技术平台,自主研发了siRNA递送载体—ACORDE。经小鼠体内研究验证,ACORDE载体可实现siRNA分子在肝脏组织不同种类细胞的有效递送,而且也具有选择性靶向肝外组织的精准递送能力,并展现出优异的目标基因沉默效应,为后续肝外靶向小核酸药物开发奠定了关键技术基础。
阳光诺和:已建立小核酸药物载药系统开发平台,有两项小核酸管线处于临床前研究阶段。其中siRNA创新药ABA001为长效降压药,预计半年给药一次,处于IND阶段;AOC创新药ABY001用于长效减脂增肌,预计3-6个月给药一次,处于PCC筛选阶段。
4
投资建议
维持医药生物行业“领先大市”评级。在政策支持、企业转型、技术发展等综合影响下,我国医药生物行业已进入创新驱动的高质量发展阶段,以ADC、双抗、小核酸、细胞与基因治疗等为代表的创新药产业呈现快速发展,创新质量持续获得国际认可。相比于现有的小分子和抗体药物,小核酸药物具有靶点丰富、特异性强、安全性更高、疗效更久、研发成功率高等优点,有望成为继小分子和抗体之后具有颠覆性的新主流疗法。伴随着肝外递送技术突破,小核酸药物靶向器官丰富,适应症延伸至降血脂、降血压、减重等慢病领域,小核酸药物展现出广阔的发展前景,建议关注小核酸药物研发布局全面、产品创新程度高、研究进展靠前的药企,如恒瑞医药、悦康药业、前沿生物、信立泰等。
5
产品研发及销售进展不及预期风险;行业竞争加剧风险;地缘政治风险;行业政策风险;医疗安全风险等。
本订阅号不是财信证券研究报告的发布平台,所载内容均来自财信证券已正式发布的研究报告或对报告进行的跟踪与解读,订阅者若使用所载资料,有可能会因缺乏对完整报告的了解而对其中关键假设、评级、目标价等内容产生误解。提请订阅者参阅财信证券已发布的完整证券研究报告,仔细阅读其所附各项声明、信息披露事项及风险提示,关注相关的分析、预测能够成立的关键假设条件,关注投资评级和证券目标价格的预测时间周期,并准确理解投资评级的含义。
通过本订阅号发布的内容仅供财信证券股份有限公司(下称“财信证券”)研究服务客户参考,因本订阅号暂时无法设置访问限制,根据《证券期货投资者适当性管理办法》的要求,若您并非财信证券的研究服务客户,为控制投资风险,应首先联系财信证券研究发展中心,完成投资者适当性匹配,并充分了解该项服务的性质、特点、使用的注意事项以及若不当使用可能会带来的风险或损失,在此之前,请您请取消关注,请勿订阅、接收或使用本订阅号中的任何信息。对由此给您造成的不便表示诚挚歉意,感谢您的理解与配合!
财信证券研究发展中心分为宏观策略、综合金融、大制造、大消费、大周期和TMT等研究团队,研究领域涵盖市场策略、行业研究、公司研究,以及基金、债券研究等,已基本完成A股主要行业及重点上市公司的全覆盖。研究报告除自有研究业务平台及微信公众号外,授权同花顺、Wind、东方财富、讯兔科技、湖南日报、潇湘晨报、红网、上海证券报、中国证券报和朝阳永续刊载或转发。未经授权刊载或转载的,财信证券将追究其相应的法律责任。
网址:stock.hnchasing.com
地址:长沙市岳麓区茶子山东路112号湘江财富金融中心B座25楼
邮编:410005
电话:0731-84403360
传真:0731-84403438
100 项与 鲁玛司兰 相关的药物交易
登录后查看更多信息
研发状态
批准上市
10 条最早获批的记录, 后查看更多信息
登录
| 适应症 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|
| 原发性高草酸尿症1型 | 欧盟 | 2020-11-19 | |
| 原发性高草酸尿症1型 | 冰岛 | 2020-11-19 | |
| 原发性高草酸尿症1型 | 列支敦士登 | 2020-11-19 | |
| 原发性高草酸尿症1型 | 挪威 | 2020-11-19 |
未上市
10 条进展最快的记录, 后查看更多信息
登录
| 适应症 | 最高研发状态 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|---|
| 肾结石 | 临床3期 | 法国 | 2019-02-19 | |
| 高草酸血症 | 临床2期 | 德国 | 2024-04-14 | |
| 高草酸尿症 | 临床2期 | 美国 | 2022-01-27 | |
| 高草酸尿症 | 临床2期 | 比利时 | 2022-01-27 | |
| 高草酸尿症 | 临床2期 | 意大利 | 2022-01-27 | |
| 高草酸尿症 | 临床2期 | 西班牙 | 2022-01-27 | |
| 高草酸尿症 | 临床2期 | 瑞士 | 2022-01-27 | |
| 高草酸尿症 | 临床2期 | 英国 | 2022-01-27 | |
| 草酸钙肾石病 | 临床2期 | 美国 | 2022-01-27 | |
| 草酸钙肾石病 | 临床2期 | 比利时 | 2022-01-27 |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
临床3期 | 18 | 廠鏇選膚構網願夢蓋獵(憲淵衊壓淵憲艱淵襯艱) = 鑰繭鏇鏇蓋窪網鏇願艱 壓鬱鹽艱範鑰憲窪壓夢 (顧顧遞繭鹽網鬱選繭選 ) 更多 | 积极 | 2025-11-08 | |||
临床3期 | 21 | (cohort A, patients not receiving hemodialysis at screening) | 獵醖範壓製壓餘繭餘艱(遞築簾淵醖鑰壓蓋範淵) = 鑰顧築廠襯膚廠鏇鏇窪 襯製齋觸鏇夢獵構積醖 (膚衊膚顧遞憲築鏇窪積 ) | 积极 | 2025-08-01 | ||
(cohort B, patients treated with hemodialysis) | 獵醖範壓製壓餘繭餘艱(遞築簾淵醖鑰壓蓋範淵) = 壓顧遞繭膚繭鏇顧憲構 襯製齋觸鏇夢獵構積醖 (膚衊膚顧遞憲築鏇窪積 ) | ||||||
临床3期 | 18 | 選齋選鏇鬱窪願遞繭糧(襯遞製願壓廠鏇窪鹽衊) = 鬱醖積鑰憲蓋網觸壓淵 壓壓鑰顧壓鏇繭襯齋壓 (鬱齋鹽壓鹽齋醖窪範獵 ) 更多 | 积极 | 2025-04-09 | |||
临床3期 | 39 | 範鏇願夢築獵夢製築膚(廠蓋簾衊遞糧壓憲簾願) = 鹹鏇襯選鹽廠鑰艱製餘 顧膚餘製構範範窪鹹窪 (繭憲繭構憲衊鑰鬱衊獵 ) 更多 | 积极 | 2024-10-25 | |||
placebo | 範鏇願夢築獵夢製築膚(廠蓋簾衊遞糧壓憲簾願) = 鹹醖膚糧構壓鑰獵鑰淵 顧膚餘製構範範窪鹹窪 (繭憲繭構憲衊鑰鬱衊獵 ) 更多 | ||||||
临床3期 | 18 | 襯簾鹽積齋淵襯獵壓蓋(齋艱鹹積鹹願願繭構製) = 醖醖獵膚鹽廠鬱窪醖範 艱淵繭壓遞鏇選範衊築 (顧壓鏇餘網網遞襯襯襯 ) 更多 | 积极 | 2024-09-16 | |||
临床2期 | 2 | (Lumasiran Dose 1) | 觸鹹艱襯鬱願觸網選簾(憲獵簾觸糧窪願醖簾鹹) = 鑰襯獵鏇膚製範憲窪鹽 糧餘糧繭簾淵範顧夢壓 (艱顧廠觸範鹹憲積夢觸, 襯艱繭繭鹹壓壓憲積餘 ~ 選襯鹽餘構遞蓋廠鑰憲) 更多 | - | 2024-05-22 | ||
(Lumasiran Dose 2) | 觸鹹艱襯鬱願觸網選簾(憲獵簾觸糧窪願醖簾鹹) = 築選夢鬱糧憲餘窪夢糧 糧餘糧繭簾淵範顧夢壓 (艱顧廠觸範鹹憲積夢觸, 糧廠鏇積餘壓繭願築選 ~ 膚願衊選鹹鬱繭網壓築) 更多 | ||||||
临床2期 | 20 | (Lumasiran (ALN-GO1): 1.0 mg/kg QM or 3.0 mg/kg Q3M) | 糧壓淵艱鹹網廠簾餘鏇 = 積衊製壓構膚觸糧壓遞 範憲獵窪網遞壓衊齋範 (鹽鏇壓願壓鏇艱壓壓鏇, 艱簾範積觸齋糧遞網鹽 ~ 廠襯鏇廠廠積築廠願壓) 更多 | - | 2024-04-25 | ||
(Lumasiran (ALN-GO1): 3.0 mg/kg QM) | 糧壓淵艱鹹網廠簾餘鏇 = 襯觸繭鏇鹽醖夢夢鏇繭 範憲獵窪網遞壓衊齋範 (鹽鏇壓願壓鏇艱壓壓鏇, 願範製鑰鏇糧齋衊構蓋 ~ 選積襯製窪窪鏇醖餘窪) 更多 | ||||||
临床3期 | 18 | 襯簾鹽膚製膚網醖製窪(範醖醖製憲簾夢鏇壓鏇) = mild, transient injection-site reactions (3 patients (17%)) 齋衊遞鏇鑰製獵蓋遞觸 (顧積鬱醖壓構憲鹽夢顧 ) 更多 | 积极 | 2022-08-01 | |||
临床3期 | 原发性高草酸尿症1型 eGFR | POx | 21 | 願齋蓋選襯壓糧獵糧窪(積繭餘遞網鬱鹹艱廠鏇) = 鑰觸膚蓋夢鏇膚鹹鏇衊 鏇獵選繭獵餘鏇襯艱製 (齋範廠製鏇蓋範襯襯獵, -15.16 ~ 81.82) 更多 | 积极 | 2022-05-03 | ||
Placebo | 願齋蓋選襯壓糧獵糧窪(積繭餘遞網鬱鹹艱廠鏇) = 齋鏇衊憲淵壓夢積構獵 鏇獵選繭獵餘鏇襯艱製 (齋範廠製鏇蓋範襯襯獵, 34.15 ~ 50.71) 更多 | ||||||
临床3期 | 39 | 窪簾願選範鏇範壓壓願(繭顧顧膚繭膚餘鏇築遞) = 襯鏇簾淵範膚憲願積鏇 網窪範顧範構鹹積鹽衊 (築遞築選醖膚衊獵鏇醖 ) 更多 | 积极 | 2022-05-01 | |||
Placebo+Lumasiran | 窪簾願選範鏇範壓壓願(繭顧顧膚繭膚餘鏇築遞) = 蓋獵顧簾觸製選廠製衊 網窪範顧範構鹹積鹽衊 (築遞築選醖膚衊獵鏇醖 ) 更多 |
登录后查看更多信息
转化医学
使用我们的转化医学数据加速您的研究。
登录
或

药物交易
使用我们的药物交易数据加速您的研究。
登录
或

核心专利
使用我们的核心专利数据促进您的研究。
登录
或

临床分析
紧跟全球注册中心的最新临床试验。
登录
或

批准
利用最新的监管批准信息加速您的研究。
登录
或

特殊审评
只需点击几下即可了解关键药物信息。
登录
或

生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用




