预约演示
更新于:2026-02-28
JMB-2002
更新于:2026-02-28
概要
基本信息
药物类型 单克隆抗体 |
别名 anti-SARS-CoV-2 neutralizing antibody、JMB2002、MB-2002 |
作用方式 抑制剂 |
作用机制 SARS-CoV-2 S protein抑制剂(冠状病毒刺突糖蛋白抑制剂) |
在研适应症 |
非在研适应症- |
非在研机构- |
权益机构- |
最高研发阶段临床2期 |
首次获批日期- |
最高研发阶段(中国)临床2期 |
特殊审评- |
登录后查看时间轴
关联
1
项与 JMB-2002 相关的临床试验ChiCTR2100042150
Phase I clinical study to evaluate the safety, tolerability, pharmacokinetic profile and immunogenicity of JMB-2002 injection in Chinese healthy subjects after intravenous infusion of single dose
开始日期2021-01-12 |
申办/合作机构- |
100 项与 JMB-2002 相关的临床结果
登录后查看更多信息
100 项与 JMB-2002 相关的转化医学
登录后查看更多信息
100 项与 JMB-2002 相关的专利(医药)
登录后查看更多信息
10
项与 JMB-2002 相关的文献(医药)2022-07-01·ARCHIVES OF PATHOLOGY & LABORATORY MEDICINE3区 · 医学
Anti–Receptor-Binding Domain Immunoglobulin G Antibody as a Predictor of Seropositivity for Anti–SARS-CoV-2 Neutralizing Antibody
3区 · 医学
Article
作者: Li, Wei ; Liu, Li-Li ; Niu, Jian-Jun ; Li, Qiu-Ling ; Xu, Qiu-Yan ; Wang, Yong-Jing ; Xue, Jian-Hang
Context.—:
Neutralizing antibody detection can assess the incidence of COVID-19 and the effectiveness of vaccines. However, commercial reagents for neutralizing antibodies were developed after the anti–SARS-CoV-2 immunoglobulin (Ig) G and IgM antibodies. Therefore, some laboratories did not perform neutralizing antibody testing services because of multiple factors.
Objective.—:
To find a fast, accurate, and economic alternative for the detection of neutralizing antibodies for the development of COVID-19 screening programs.
Design.—:
The response and correlation of 3 antibodies (anti–spike protein neutralizing antibody, total anti–receptor-binding domain [RBD] antibody, and anti-RBD IgG) were determined by observing the dynamics in 61 participants for 160 days after vaccination.
Results.—:
The levels of neutralizing and anti-RBD IgG antibodies reached their peak values on day 42 after vaccination (120.75 IU/mL and 14.38 signal-to-cutoff ratio [S/CO], respectively). The total antibody levels peaked at 138.47 S/CO on day 35 after vaccination. The strongest correlation was found between neutralizing and anti-RBD IgG antibody levels (r = 0.894, P < .001). The area under the receiver operating characteristic curve for total antibody levels for the prediction of seropositivity for neutralizing antibodies was 0.881 (P < .001), and that for anti-RBD IgG antibody levels was 0.937 (P < .001).
Conclusions.—:
Neutralizing and anti-RBD IgG antibody levels were strongly correlated, and thus anti-RBD IgG antibody levels can be used for the accurate assessment of immunity following SARS-CoV-2 infection or vaccination.
2021-10-01·Transfusion3区 · 医学
How do I… facilitate a rapid response to a public health emergency requiring plasma collection with a public–private partnership?
3区 · 医学
Article
作者: Miller, Maureen J. ; Duvenhage, Michael ; Holbrook, Michael ; Skrzekut, Adam ; Kracalik, Ian ; Konkle, Barbara A. ; Lofy, Kathryn H. ; Jones, Jefferson M. ; Basavaraju, Sridhar V. ; Haley, N. Rebecca ; Higgs, Elizabeth ; Bonnett, Tyler ; Paranjape, Suman
Abstract:
In March 2020, there were no treatment options for COVID‐19. Passive immune therapy including anti‐SARS‐CoV‐2 hyperimmune globulin (hIVIG) was a logical candidate for COVID‐19 therapeutic trials, given past success treating emerging pathogens with endogenous neutralizing antibodies. We established a plasma collection protocol for persons recovered from COVID‐19. To speed recruitment in the first U.S. hotspot, Seattle, Washington, federal and state public health agencies collaborated with Bloodworks Northwest to collect convalescent plasma (CP) for manufacturing hIVIG. During March–December 2020, we identified and recruited prospective CP donors via letters to persons recovered from COVID‐19 with laboratory‐confirmed SARS‐CoV‐2 infection. Prospective donors were pre‐screened and administered a medical history survey. Anti‐SARS‐CoV‐2 neutralizing antibody (NAb) titers were classified as qualifying (≥1:80) or non‐qualifying (<1:80) for enrollment based on a live virus neutralization assay. Generalized estimating equations were used to identify characteristics of donors associated with qualifying versus nonqualifying NAb titers. Overall, 21,359 letters resulted in 3207 inquiries, 2159 prescreenings with laboratory‐confirmed SARS‐CoV‐2 infection, and 573 donors (27% of all pre‐screenings with confirmed infection) who provided a screening plasma donation. Of 573 donors screened, 254 (44%) provided plasma with qualifying NAb titers, resulting in 1284 units for hIVIG manufacture. In a multivariable model, after adjusting for other factors, time (60 days) from COVID‐19 symptom onset to screening was associated with lower odds of qualifying NAb (adjusted odds ratio = 0.67, 95% CI: 0.48–0.94). The collaboration facilitated a rapid response to develop and provide hIVIG for clinical trials and CP for transfusion. Only 1 in 12 donor inquiries resulted in a qualifying plasma donation. Challenges included recruitment and the relatively low percentage of persons with high NAb titers and limited screening capacity. This resource‐intensive collaboration may not be scalable but informs preparedness and response strategies for plasma collection in future epidemics. Operational readiness plans with templates for screening, consent, and data collection forms are recommended.
2020-01-01·mAbs2区 · 医学
Anti-SARS-CoV-2 neutralizing monoclonal antibodies: clinical pipeline
2区 · 医学
Article
作者: Valdiserra, Giulia ; Ferraro, Sara ; Blandizzi, Corrado ; Tuccori, Marco ; Maggi, Fabrizio ; Cappello, Emiliano ; Focosi, Daniele ; Convertino, Irma
Monoclonal antibody (mAb) therapy has been previously exploited for viral infections, such as respiratory syncytial virus pneumonia and Ebolavirus disease. In the ongoing COVID-19 pandemic, early signals of efficacy from convalescent plasma therapy have encouraged research and development of anti-SARS-CoV-2 mAbs. While many candidates are in preclinical development, we focus here on anti-SARS-CoV-2 neutralizing mAbs (or mAb cocktails) that represent the late-stage clinical pipeline, i.e., those currently in Phase 2 or Phase 3 clinical trials. We describe the structure, mechanism of action, and ongoing trials for VIR-7831, LY-CoV555, LY-CoV016, BGB-DXP593, REGN-COV2, and CT-P59. We speculate also on the next generation of these mAbs.
10
项与 JMB-2002 相关的新闻(医药)2023-03-27
·生物谷
商业化8个月后,首款国产新冠中和抗体疗法官宣停产。
商业化8个月后,首款国产新冠中和抗体疗法官宣停产。
3月24日,腾盛博药发布公告称,决定停止生产旗下控股子公司腾盛华创医药技术(北京)有限公司(下称“腾盛华创”)研发的新冠病毒中和抗体联合治疗药物安巴韦单抗注射液(amubarvimab,BRII-196)及罗米司韦单抗注射液(romlusevimab,BRII-198)。
公告进一步指出,这一决定是基于不断演变的新冠疫情趋势和相关政策的更新,包括美国卫生与公众服务部(HHS)计划于2023年5月结束新冠疫情国家紧急状态和公共卫生紧急状态(PHE),以及医药合同定制研发生产企业(CDMO)对药物相关情况的长时间观察。
财报显示,2022年,腾盛博药营收由零增加至5160万元,均来自安巴韦单抗╱罗米司韦单抗联合疗法。其研发开支为4.41亿元,行政开支为人民币1.69亿元,销售及营销开支为2686万元,年内亏损约4.84亿元。
对于此次停产后,是否还有剩余库存,腾盛博药相关负责人回应《华夏时报》记者称:“我们已基本将该药物的全部适销产品销售到全国25个省份了。”
01
去年销售额5160万元
2021年12月8日,作为国产首个新冠中和抗体疗法,腾盛博药的安巴韦单抗和罗米司韦单抗联合疗法获批在国内上市,用于治疗轻型和普通型且伴有进展为重型(包括住院或死亡)高风险因素的成人和青少年(12—17岁,体重≥40 kg)新型冠状病毒感染(COVID-19)患者,其中青少年适应症人群为附条件批准。
据此前甘肃省公共资源交易中心挂网信息,安巴韦单抗和罗米司韦单抗联合疗法挂网单价为“每支不高于全国最低价2417元”,由此推算,每人治疗费用近万元。
腾盛博药表示,2022年7月在中国商业化上市后,公司已基本将安巴韦单抗╱罗米司韦单抗联合疗法的全部适销产品出售给全国25个省份及358家医院,该药在2022年实现销售收入约5160万元。作为确保人道主义通道及为遏制疫情暴发作出贡献的承诺的一部分,腾盛博药于商业化上市前向中国21个城市及22家医院捐赠近3000人份的该抗体用于紧急使用。
3月24日,腾盛博药公告称,公司已作出决定结束安巴韦单抗╱罗米司韦单抗联合疗法项目,并已停止生产工作以将资源重新转向核心项目。同时,公司预计,未来无论在中国或美国以及其他地区,都不会再从该联合疗法中产生可观收入。
财报显示,2022年,公司营收由零增加至5160万元,均来自安巴韦单抗╱罗米司韦单抗联合疗法。其研发开支为4.41亿元,行政开支为人民币1.69亿元,销售及营销开支为2686万元,年内亏损约4.84亿元。2022年底流动资产为30.77亿元,其中现金及现金等价物11.9亿元。
记者查询发现,腾盛博药拥有一条针对传染病及CNS疾病的由10多个创新候选产品组成的管线,核心候选药物为治疗乙型肝炎病毒、人类免疫缺陷病毒HIV等传染病的药物,但除安巴韦单抗╱罗米司韦单抗联合疗法外,其余均处于临床早期阶段,进入临床2期的只有4项。
进展最快的是由VBI及Vir授权的乙型肝炎项目,目前正在开展BRII-179(一种新型重组蛋白免疫疗法)和BRII-835 (一种经皮下注射给药的靶向HBV病毒RNA的siRNA药物)联合治疗乙肝的研究。财报称,2022年2月,2期MRCT联合疗法研究已完成90名亚太地区患者入组。
对于研发进展,腾盛博药坦言,“由于我们的大多数候选药物仍在进行临床试验以及COVID-19项目被终止,我们预计未来很短期内不会实现候选药物销售或商业化。2022年,我们重新关注作为该领域行业领跑者的中国HBV的核心开发项目,以及精神障碍治疗项目,目前正在美国加速精神障碍治疗相关的临床开发。”
02
停产并非个例
对于新冠中和抗体的停产,市场似乎并不感到意外。
中和抗体是指由B淋巴细胞产生的,能识别抗原特异性部位而使抗原失去活性,抑制其功能的抗体,也被称为保护性抗体。据不完全统计,目前,全球已有20款中和抗体药物获批临床,国内在研治疗新冠中和抗体药物至少有10款。
整体来看,全球已有6款中和抗体或组合疗法获得紧急使用授权,包括此前获批的再生元casirivimab/imdevimab联合疗法、礼来/君实生物合作开发的etesevimab/bamlanivimab联合疗法、葛兰素史克与Vir Biotechnology合作研发的Sotrovimab。
尽管赛道火热,但中和抗体的疗效,一直被业内质疑。
病毒学专家常荣山此前接受本报记者采访时表示,新冠病毒流行株的迭代很快,平均6个月左右。抵抗不同的变异株,需要的中和抗体(抗体特异性)是不同的,半数抑制浓度也是不同的,这两个指标能说明抗体药物的有效性,此外,维持保护时长也是一个重要指标,因此中和抗体的保护力值得关注。
事实确实如此,随着病毒不断变异,中和抗体一度因为疗效不足出现被停止使用的情况。
2022年3月28日,根据美国食品和药物管理局(FDA)的最新指示,全美有8个州将停止使用葛兰素史克和Vir Biotechnology联合研发的新冠抗体疗法。FDA声明称:“对具有高度传播性的奥密克戎BA.2变体而言,于2021年5月获得紧急使用授权(EUA)的葛兰素史克新冠抗体疗法sotrovimab可能不太有效,不适用于大规模流行的的BA.2病毒株。”
更早一些的2022年1月24日,FDA宣布限制再生元、礼来/君实新冠中和抗体的使用,因这两款抗体对Omicron突变株无效,除非患者感染的是对这些新冠中和抗体敏感的突变株如Delta,医生才可以对患者使用这两款抗体。据再生元2022年财报,自限制使用以来,REGEN-COV在美国的销售业绩瞬间降至冰点,为0销售。
或许同样是基于疗效不佳的原因,2023年1月下旬,FDA撤销了对阿斯利康Evusheld的EUA,自此,在美国投入使用的六款中和抗体全线败退。而正在排队申请EUA的中和抗体,也成为了明日黄花。
2022年4月,再生元宣布FDA延长了REGEN-COV用于治疗和预防新冠肺炎的上市申请审查,PDUFA日期是2022年7月13日,但至今仍未有更多消息披露。
2021年10月,腾盛博药就宣布其在研的中和抗体联合疗法已经向美国FDA提交了紧急使用授权(EUA)申请。如今,两年过去,等来的却是停产和撤回的消息。
“公司正在与美国FDA沟通,在完成所有监管要求后,适当的时候撤回EUA申请,并会与中国国家药品监督管理局(NMPA)进一步沟通,在2023年第三季度完成监管机构的各项要求后,撤回生物制品许可申请(BLA)。”腾盛博药相关负责人向《华夏时报》记者透露。
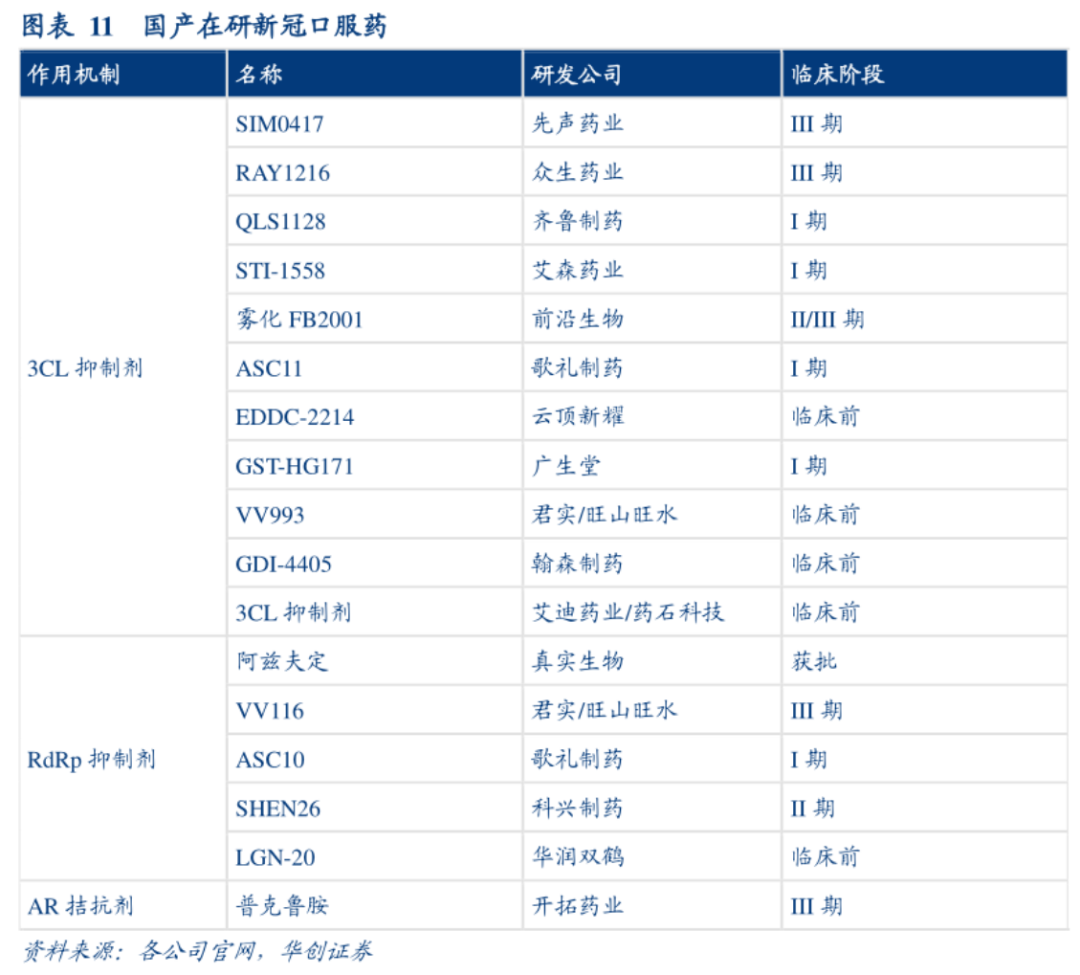
至于其他一度被热议的中和抗体资产,诸如迈威生物9MW3311 、神州细胞SCTA01、绿叶制药LY-CovMab、复宏汉霖HLX70、济民可信JMB2002等等名字,恐怕已经成为掘金路上的一个分母。
与此同时,还有一系列在研国产小分子新冠药物的命运也成疑...
来源:华夏时报
财报上市批准医药出海
2023-03-26
·药创客
商业化8个月后,首款国产新冠中和抗体疗法官宣停产。3月24日,腾盛博药发布公告称,决定停止生产旗下控股子公司腾盛华创医药技术(北京)有限公司(下称“腾盛华创”)研发的新冠病毒中和抗体联合治疗药物安巴韦单抗注射液(amubarvimab,BRII-196)及罗米司韦单抗注射液(romlusevimab,BRII-198)。公告进一步指出,这一决定是基于不断演变的新冠疫情趋势和相关政策的更新,包括美国卫生与公众服务部(HHS)计划于2023年5月结束新冠疫情国家紧急状态和公共卫生紧急状态(PHE),以及医药合同定制研发生产企业(CDMO)对药物相关情况的长时间观察。财报显示,2022年,腾盛博药营收由零增加至5160万元,均来自安巴韦单抗╱罗米司韦单抗联合疗法。其研发开支为4.41亿元,行政开支为人民币1.69亿元,销售及营销开支为2686万元,年内亏损约4.84亿元。对于此次停产后,是否还有剩余库存,腾盛博药相关负责人回应《华夏时报》记者称:“我们已基本将该药物的全部适销产品销售到全国25个省份了。”01去年销售额5160万元2021年12月8日,作为国产首个新冠中和抗体疗法,腾盛博药的安巴韦单抗和罗米司韦单抗联合疗法获批在国内上市,用于治疗轻型和普通型且伴有进展为重型(包括住院或死亡)高风险因素的成人和青少年(12—17岁,体重≥40 kg)新型冠状病毒感染(COVID-19)患者,其中青少年适应症人群为附条件批准。据此前甘肃省公共资源交易中心挂网信息,安巴韦单抗和罗米司韦单抗联合疗法挂网单价为“每支不高于全国最低价2417元”,由此推算,每人治疗费用近万元。腾盛博药表示,2022年7月在中国商业化上市后,公司已基本将安巴韦单抗╱罗米司韦单抗联合疗法的全部适销产品出售给全国25个省份及358家医院,该药在2022年实现销售收入约5160万元。作为确保人道主义通道及为遏制疫情暴发作出贡献的承诺的一部分,腾盛博药于商业化上市前向中国21个城市及22家医院捐赠近3000人份的该抗体用于紧急使用。3月24日,腾盛博药公告称,公司已作出决定结束安巴韦单抗╱罗米司韦单抗联合疗法项目,并已停止生产工作以将资源重新转向核心项目。同时,公司预计,未来无论在中国或美国以及其他地区,都不会再从该联合疗法中产生可观收入。财报显示,2022年,公司营收由零增加至5160万元,均来自安巴韦单抗╱罗米司韦单抗联合疗法。其研发开支为4.41亿元,行政开支为人民币1.69亿元,销售及营销开支为2686万元,年内亏损约4.84亿元。2022年底流动资产为30.77亿元,其中现金及现金等价物11.9亿元。记者查询发现,腾盛博药拥有一条针对传染病及CNS疾病的由10多个创新候选产品组成的管线,核心候选药物为治疗乙型肝炎病毒、人类免疫缺陷病毒HIV等传染病的药物,但除安巴韦单抗╱罗米司韦单抗联合疗法外,其余均处于临床早期阶段,进入临床2期的只有4项。进展最快的是由VBI及Vir授权的乙型肝炎项目,目前正在开展BRII-179(一种新型重组蛋白免疫疗法)和BRII-835 (一种经皮下注射给药的靶向HBV病毒RNA的siRNA药物)联合治疗乙肝的研究。财报称,2022年2月,2期MRCT联合疗法研究已完成90名亚太地区患者入组。对于研发进展,腾盛博药坦言,“由于我们的大多数候选药物仍在进行临床试验以及COVID-19项目被终止,我们预计未来很短期内不会实现候选药物销售或商业化。2022年,我们重新关注作为该领域行业领跑者的中国HBV的核心开发项目,以及精神障碍治疗项目,目前正在美国加速精神障碍治疗相关的临床开发。”02停产并非个例对于新冠中和抗体的停产,市场似乎并不感到意外。中和抗体是指由B淋巴细胞产生的,能识别抗原特异性部位而使抗原失去活性,抑制其功能的抗体,也被称为保护性抗体。据不完全统计,目前,全球已有20款中和抗体药物获批临床,国内在研治疗新冠中和抗体药物至少有10款。整体来看,全球已有6款中和抗体或组合疗法获得紧急使用授权,包括此前获批的再生元casirivimab/imdevimab联合疗法、礼来/君实生物合作开发的etesevimab/bamlanivimab联合疗法、葛兰素史克与Vir Biotechnology合作研发的Sotrovimab。尽管赛道火热,但中和抗体的疗效,一直被业内质疑。病毒学专家常荣山此前接受本报记者采访时表示,新冠病毒流行株的迭代很快,平均6个月左右。抵抗不同的变异株,需要的中和抗体(抗体特异性)是不同的,半数抑制浓度也是不同的,这两个指标能说明抗体药物的有效性,此外,维持保护时长也是一个重要指标,因此中和抗体的保护力值得关注。事实确实如此,随着病毒不断变异,中和抗体一度因为疗效不足出现被停止使用的情况。2022年3月28日,根据美国食品和药物管理局(FDA)的最新指示,全美有8个州将停止使用葛兰素史克和Vir Biotechnology联合研发的新冠抗体疗法。FDA声明称:“对具有高度传播性的奥密克戎BA.2变体而言,于2021年5月获得紧急使用授权(EUA)的葛兰素史克新冠抗体疗法sotrovimab可能不太有效,不适用于大规模流行的的BA.2病毒株。”更早一些的2022年1月24日,FDA宣布限制再生元、礼来/君实新冠中和抗体的使用,因这两款抗体对Omicron突变株无效,除非患者感染的是对这些新冠中和抗体敏感的突变株如Delta,医生才可以对患者使用这两款抗体。据再生元2022年财报,自限制使用以来,REGEN-COV在美国的销售业绩瞬间降至冰点,为0销售。或许同样是基于疗效不佳的原因,2023年1月下旬,FDA撤销了对阿斯利康Evusheld的EUA,自此,在美国投入使用的六款中和抗体全线败退。而正在排队申请EUA的中和抗体,也成为了明日黄花。2022年4月,再生元宣布FDA延长了REGEN-COV用于治疗和预防新冠肺炎的上市申请审查,PDUFA日期是2022年7月13日,但至今仍未有更多消息披露。2021年10月,腾盛博药就宣布其在研的中和抗体联合疗法已经向美国FDA提交了紧急使用授权(EUA)申请。如今,两年过去,等来的却是停产和撤回的消息。“公司正在与美国FDA沟通,在完成所有监管要求后,适当的时候撤回EUA申请,并会与中国国家药品监督管理局(NMPA)进一步沟通,在2023年第三季度完成监管机构的各项要求后,撤回生物制品许可申请(BLA)。”腾盛博药相关负责人向《华夏时报》记者透露。至于其他一度被热议的中和抗体资产,诸如迈威生物9MW3311 、神州细胞SCTA01、绿叶制药LY-CovMab、复宏汉霖HLX70、济民可信JMB2002等等名字,恐怕已经成为掘金路上的一个分母。与此同时,还有一系列在研国产小分子新冠药物的命运也成疑...识别微信二维码,添加小编,符合条件者即可加入生物制品圈微信群!请注明:姓名+单位+研究方向! End 声明:本文来源华夏时报,旨在传递更多信息,版权归原作者所有。原创文章转载均需经过授权并注明来源,如涉及内容、版权或其他问题请时联系小编删除!文章仅代表作者个人观点,并不代表公众号立场。本公众号拥有对此声明的最终解释权!投稿邮箱:yck19876@163.com
财报
2023-02-20
·药智网
腾盛博药股价的持续闪崩,让束之高阁的新冠中和抗体概念股又重回聚光灯下。与“新冠概念股”过山车般起伏不同,昙花一现的中和抗体概念似乎江河日下的态势不可逆转。随着新冠口服治疗药物的百花齐放,似乎再也没有留下空隙让中和抗体打一场漂亮的“翻生仗”。新冠中和抗体的未来,在哪里?图1 腾盛博药股价走势图片来源:百度股市通曾经百亿高光,目前全线败退时间回拨至2020年,疫情不确定性带来的旺盛需求、多管齐下的治疗策略以及百花齐放的创新研发,带动了整个新冠治疗产业链概念板块的上涨。在第一道防线新冠疫苗始终无法有效阻隔疫情的快速传播,而新药口服“特效药”又迟迟不见踪影的局促背景下,新冠中和抗体的高光时刻闪耀登场。顾名思义,中和抗体便是能发挥中和作用的抗体。作为一种RNA病毒,新冠病毒主要利用S蛋白,具体地说是蛋白上的受体结合域(RBD),与人体细胞表面受体ACE2结合,进而突破细胞屏障,实现其在人体的复制、传播和感染。而新冠中和抗体的存在,就是致力于切断上述过程,一旦病原体不能继续感染细胞,疾病进展就会得到控制,而剩余的病原体也会被机体慢慢清除,最终达到治愈。与疫苗和口服治疗药物相比,中和抗体药物的研发周期要短得多。如果一切顺利,8-12个月就有望研发成功,甚至可能更快。赶在疫情结束之前,多家跨国药企成功抢滩登陆,而且一上市就成为“印钞机”。2021年,再生元新冠中和抗体收入达到75.74亿美元,礼来也有22.39亿美元……全球销售额累计超百亿美金!美国FDA先后给6款新冠中和抗体或联合疗法紧急使用授权(EUA),那时候的中和抗体一度被誉为“新冠终结的曙光”。然而,这个高光时刻,来得快去得也快。随着今年1月下旬,仅有的Evusheld也未能幸免,先后获得EUA的新冠中和抗体或联合疗法,全部叫停,EUA均被撤销。至此,一度热闹的中和抗体赛道,变得门可罗雀。表1 被FDA授权及限制的新冠中和抗体统计在中国,新冠中和抗体的剧本也未能逃脱悲剧的命运。2021年12月,腾盛博药的安巴韦单抗/罗米思韦单抗联合疗法获得NMPA批准上市,成为国内首个在中国获批上市的中和抗体,至今也是中国目前唯一一个。腾盛博药的股价,一度突破45港元/股。然后,还没正式享受新冠红利的腾盛博药,就即将面临着多款中和抗体疗法遭限制使用或撤销EUA的重大考验。期望获得美国市场“入场券”的念想,终究在口服药上市的征程中败下阵来。腾盛博药的股价一路“跌跌不休”,接近90%的股价跌没,目前只剩下6块多港元。为何中和抗体会成为明日黄花?曾经凭借着速度先发优势“一发冲天”的中和抗体,为何稍瞬即逝?一方面,正所谓出生决定命运,中和抗体便是如此。中和抗体主要的作用位点是S蛋白,让人揪心的是,这个区域是高度可变的。病毒在持续变异过程中,就能够逃避抗体反应。图2 中和抗体作用示意图图片来源:喀斯玛商城躲不过的免疫逃逸,导致大多数中和抗体快速失去战斗力。不能只怪中和抗体效力不行,谁让新冠病毒太“狡猾”。另外一方面,中和抗体的性价比注定它只是“阶段性产物”。中和抗体的制备过程较复杂,一般需要通过对患者的B淋巴细胞进行单细胞测序,用新冠蛋白的刺突蛋白与细胞相互作用,筛选出高亲和力的中和抗体,继而通过基因工程对抗体进行调整、优化、克隆,以完成中和抗体的筛选和大规模生产。算下来,得到中和抗体的时间成本和资金成本,都不会太低。这导致中和抗体的产能有限,不能满足全球抗疫的需求。产能不足之外,新冠中和抗体的售价也不便宜。以再生元的中和抗体为例,患者一个疗程的费用为2100美元。腾盛博药的安巴韦单抗/罗米思韦单抗联合疗法定价也在每人份一万元人民币。如此昂贵的的价格,让中和抗体目前只能做有钱人的希望,而非人民的希望。在新冠治疗药物有限的情况下,中和抗体是首选,但在有更为有效、具有更高性价比的注射液和口服药诞生的情况下,中和抗体的地位就大不如前了。口服药上市后,多国的新冠治疗推荐指南,也首选口服药,其次才是中和抗体。而且,相比疫苗及中和抗体,口服药无惧突变,无论病毒如何变异,打击部位仍然存在,称得上是“新冠终结者”,完成阶段性使命的中和抗体,注定会被淘汰。热潮褪去的中和抗体,路在何方?据不完全统计,目前,全球已有20款中和抗体药物获批临床,国内在研治疗新冠中和抗体药物至少有10款。上市的新冠中和抗体的窗口期都结束了,其他还在路上的中和抗体资产,诸如迈威生物ABP-300、神州细胞SCTA01、信达生物的IBI314、复宏汉霖HLX70、济民可信JMB2002等等名字,恐怕也大多沦为这趟前赴后继“掘金之旅”的分母式存在。表2 中国企业参与的新冠中和抗体在研管线压重注布局中和抗体赛道的企业,该何去何从?是继续死磕,找到能跟上病毒突变速度的理想抗体?是及早离场,承认曾经的热血但终归散场的止损?还是基于自身专长而灵活换道?MNC已纷纷做出选择,对于尚在场内的本土药企也不例外。Vir Biotechnology公司和多家合作研究机构共同发布在《自然》杂志的科学论文,系统性地探索了多种针对新冠病毒刺突蛋白受体结合域(RBD)的中和抗体针对不同类型的冠状病毒的中和能力,发现了一款“广谱”中和抗体,它能够对Sarbe冠状病毒亚属(Sarbecovirus)的广泛病毒种类产生中和效力,有望为设计出更为“广谱”的中和抗体和疫苗提供洞见。“广谱”的中和抗体不失为一条迁移的路径,毕竟病毒性感染带来的疾病一直是人类心头的痛。此外,被动免疫的想法,对其他领域也有指导价值。以RSV为例,阿斯利康就联合赛诺菲开发出首款可广泛应用于婴儿群体的RSV长效单抗Beyfortus。面对RSV疫苗长期缺位,Beyfortus或将实现社会价值和商业化的双赢。近年来,科学界对于埃博拉、HIV等疾病的研究中,抗体药物都显示出了良好的疗效和安全性,新冠中和抗体的基础有望在新领域赋予加速度。小结新冠凶猛,新冠观念股的造富“钞能力”惊人,但疫情带来的终究是阶段性红利。阶段性周期股的宿命,是情绪高点曾经有多疯狂,最后散场后的一地鸡毛就有多么落寞。无论是中和抗体、核酸检测、抗原、还是口服特效药……我们无法得知,每一个当红概念股的明天,会不会是落幕黄花的今天。但是我们可以清晰认识到,医药行业原本的生长脉络,是真正有效解决临床需求。在合适的时间,有合适的产品,以合适的价格,送达到患者手中。对于新冠中和抗体的药企而言,或进或退,所剩的时间都不多了。面对市场的起伏,该如何浮沉?考验着企业的眼光与魄力。声明:本内容为作者独立观点,不代表药智网立场。如需转载,请务必注明文章作者和来源。对本文有异议或投诉,请联系maxuelian@yaozh.com。责任编辑 | 渡转载开白 | 马老师 18323856316(同微信) 阅读原文,是昨天最受欢迎的文章哦
疫苗紧急使用授权
100 项与 JMB-2002 相关的药物交易
登录后查看更多信息
研发状态
10 条进展最快的记录, 后查看更多信息
登录
| 适应症 | 最高研发状态 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|---|
| 新型冠状病毒感染 | 临床2期 | 中国 | - |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
No Data | |||||||
登录后查看更多信息
转化医学
使用我们的转化医学数据加速您的研究。
登录
或

药物交易
使用我们的药物交易数据加速您的研究。
登录
或

核心专利
使用我们的核心专利数据促进您的研究。
登录
或

临床分析
紧跟全球注册中心的最新临床试验。
登录
或

批准
利用最新的监管批准信息加速您的研究。
登录
或

生物类似药
生物类似药在不同国家/地区的竞争态势。请注意临床1/2期并入临床2期,临床2/3期并入临床3期
登录
或

特殊审评
只需点击几下即可了解关键药物信息。
登录
或

生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用