预约演示
更新于:2025-12-27
DB-1418
更新于:2025-12-27
概要
基本信息
原研机构 |
非在研机构- |
最高研发阶段临床1/2期 |
首次获批日期- |
最高研发阶段(中国)临床申请批准 |
特殊审评快速通道 (美国) |
登录后查看时间轴
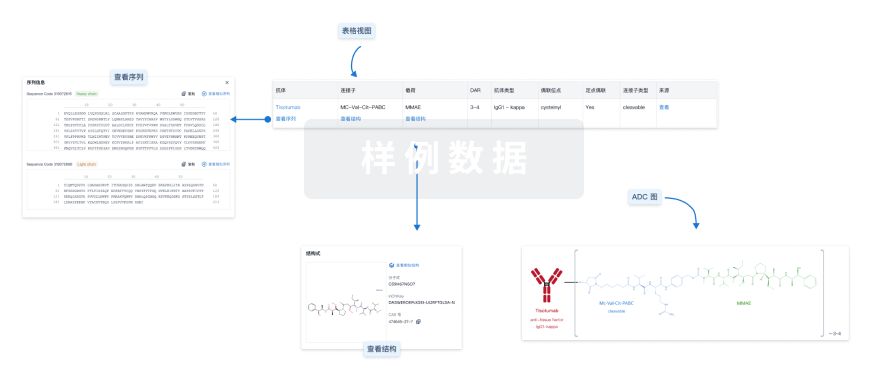
结构/序列
使用我们的ADC技术数据为新药研发加速。
登录
或
关联
1
项与 DB-1418 相关的临床试验NCT07038343
A Phase 1/2, First-in-human Study to Assess the Safety, Tolerability, Pharmacokinetics, Pharmacodynamics, and Antitumor Activity of AVZO-1418 as a Single Agent and in Combination Therapy in Patients With Locally Advanced or Metastatic Solid Tumors
This study, the first clinical trial of AVZO-1418, aims to determine the safety, tolerability, pharmacokinetics, pharmacodynamics, maximum tolerated dose, and antitumor activity of AVZO-1418 when administered intravenously as a monotherapy and potentially in combination therapy to patients with locally advanced or metastatic epithelial solid tumors.
开始日期2025-06-04 |
申办/合作机构 |
100 项与 DB-1418 相关的临床结果
登录后查看更多信息
100 项与 DB-1418 相关的转化医学
登录后查看更多信息
100 项与 DB-1418 相关的专利(医药)
登录后查看更多信息
94
项与 DB-1418 相关的新闻(医药)2025-12-09
化疗是癌症患者的常用治疗方法之一。但是,化疗副作用严重,在杀伤癌细胞的同时,也对人体正常细胞造成严重损伤。而抗体药物偶联物(ADC)的出现,如同携带精确制导系统的“生物导弹”,实现了对癌细胞的精准定点清除。
关于ADC药物
抗体-药物偶联物(ADCs)由单克隆抗体(mAb)通过连接子与细胞毒性小分子药物(即“有效载荷”)连接组成。
抗体偶联药物(ADC)可被喻为治疗中的“特洛伊木马”,因其能将抗癌药物直接递送至肿瘤内部。ADC中的抗体对特定靶点抗原具有高亲和力与强结合力,其肿瘤抗原识别位点负责介导这些相互作用。毒性有效载荷通过精心设计的连接子与抗体偶联,确保药物在血液循环中保持稳定,同时在抵达靶向癌细胞后实现载荷的可控释放。
ADC的概念并非新兴事物。早在1913年,保罗·埃尔利希便提出了这一设想,但经过数十年的研究探索,ADC才得以实现。
部分已获批与在研ADC药物
抗体偶联药物(ADC)能够靶向超过50种已知的不同抗原,其中主要靶点包括ERBB2、CD33、CD19、CD22、TROP2、HER2、MSLN(间皮素)、CLDN18.2等。其核心理念既精妙又复杂:通过精心设计的连接子将抗体与细胞毒性药物结合,从而精准地将药物导向肿瘤靶点。目前全球已获批数十款ADC药物,覆盖血液肿瘤和实体瘤。以下列举部分药物及其靶点:
维恩妥尤单抗(备思复,PADCEV,enfortumab vedotin)是一款靶向Nectin-4的ADC药物,已经获批用于局部晚期或转移性尿路上皮癌(la/mUC)成年患者。
Gemtuzumabozogamicin(吉妥珠单抗,MYLOTARG)是一种靶向CD33的ADC药物,已获批用于治疗成人新诊断的CD33阳性急性髓细胞性白血病(AML)。
索米妥昔单抗注射液(爱拉赫/ELAHERE)是一款针对叶酸受体α(FRα)靶点的ADC创新药,已获批用于既往接受过1-3线系统性治疗的叶酸受体α阳性的铂类耐药卵巢癌(PROC)。
芦康沙妥珠单抗(sac-TMT)是一款新型人滋养层细胞表面抗原2(TROP2)抗体药物偶联物(ADC),获批用于治疗既往至少接受过2种系统治疗(其中至少1种治疗针对晚期或转移性阶段)的不可切除的局部晚期或转移性三阴性乳腺癌(TNBC)成人患者。
维替索妥尤单抗(TIVDAK,tisotumab vedotin)是一种针对细胞表面组织因子(TF)的ADC药物,已获批用于治疗化疗期间或之后出现疾病进展的复发性或转移性宫颈癌成年患者。
德曲妥珠单抗(优赫得,DS-8201)是一种靶向人表皮生长因子受体2(HER2)的抗体偶联药物(ADC),已获批用于乳腺癌、胃癌以及非小细胞肺癌。
注射用维贝柯妥塔单抗(美佑恒®,MRG003)是一款靶向EGFR的ADC药物,已获批用于鼻咽癌。
除却已获批的ADC药物,目前仍有很多在研药物,部分如下:
DB-1418/AVZO-1418是一种针对EGFR/HER3双靶点的抗体药物偶联物(ADC),临床前研究中显示出对肿瘤细胞更高的结合亲和力,并已证明其在多种实体瘤中的潜在疗效,包括对EGFR耐药、EGFR低表达或HER3耐药的肿瘤。
HS-20093是一款靶向B7-H3的ADC药物,在复发或难治性肉瘤、铂耐药卵巢癌(PROC)中展现出广泛的应用潜力。
ATG-022是一款靶向CLDN18.2的ADC药物,已在临床试验中证明其疗效的深度和持久性。
癌症治疗的“导航坐标”
从已上市及正在研究中的ADC药物中,我们可以清晰地看到每一个成功ADC的背后,都站立着一个被精准定义的分子靶点。 这些靶点,如HER2、TROP2、CLDN18.2等,不仅是药物设计的“导航坐标”,更是区分患者能否获益的“分子身份证”。
HER2:人表皮生长因子受体-2 (HER2)是一种跨膜酪氨酸激酶受体。多年前被发现在人乳腺癌细胞系中扩增,并且这种扩增被证明在乳腺癌的发病机制和进展中很重要。它的主要功能是接收生长信号,调控细胞的增殖、分化和存活。而针对这种快速生长肿瘤的新型药物疗效显著。
Trop2:滋养层细胞表面抗原 2(Trop2)是一种糖蛋白,在多种实体肿瘤均有表达,包括:肺癌、乳腺癌、宫颈癌、结直肠癌、食管癌、胃癌、口腔鳞状细胞癌、卵巢癌、胰腺癌、前列腺癌、胃癌、甲状腺癌和膀胱癌。
Claudin18.2:Claudin18.2(CLDN18.2)是一种紧密连接分子,主要存在于非恶性胃上皮中,在恶性转化过程中可以在肿瘤细胞表面接近,可以参与肿瘤细胞的增殖、分化和迁移,在各种实体瘤患者中得到了广泛的研究,尤其是在胃肠道癌症患者中,为癌症治疗提供了一个有吸引力的靶点。
HER-3:HER-3是一种关键的酪氨酸蛋白激酶,是神经调节蛋白的重要细胞受体。它参与骨髓细胞分化,在正常发育和其它重要细胞过程中均发挥重要作用。
Nectin-4:Nectin-4是免疫球蛋白超家族Nectin成员中的I型跨膜糖蛋白,兼具同嗜性与异嗜性(与Nectin-1结合)细胞黏附功能,特异性表达于胎盘、胚胎组织及乳腺癌中。
CD20:一种存在于B细胞发育过程中表面的受体;通常由白血病、淋巴瘤和多发性骨髓瘤细胞表达。
CD30:一种在某些类型激活免疫细胞上表达的受体;通常由白血病和淋巴瘤细胞表达。
以下临床数据直观地展示了靶向上述靶点的ADC药物的临床效果。
尿路上皮癌:靶向Nectin-4,生存期显著延长
EV-301试验结果显示:维恩妥尤单抗的总缓解率(ORR)为40.6%,疾病控制率(DCR)为71.9%,化疗组分别为17.9%和53.4%。维恩妥尤单抗的中位总生存期(OS)为12.88个月,而化疗组为8.97个月。1年总生存率分别为51.5%和39.2%。
胃癌:CLDN18.2靶向治疗展现深度缓解
ATG-022的I/II期临床研究的最新研究结果显示:在CLDN18.2中高表达(IHC 2+ > 20%)的胃癌/胃食管结合部癌(GC/GEJC)患者中,ATG-022在两个剂量组(1.8mg/kg和2.4mg/kg)均取得了40%的客观缓解率(ORR)和约90%的疾病控制率(DCR),中位无进展生存期(PFS)达到6.97个月,12个月生存率为66.2%。
在CLDN18.2低表达及极低表达(IHC 2+≤20%)的患者中,客观缓解率(ORR)依然达到了33.3%,包括1例完全缓解(CR)和5例部分缓解(PR),达到完全缓解(CR)的患者参与研究已超过22个月。
鼻咽癌:EGFR靶向ADC联合免疫,实现高缓解与长生存
2025年欧洲肿瘤内科学会(ESMO)大会上公布了MRG003联合PD-1单抗(普特利单抗)的II期临床试验结果。证明了MRG003在鼻咽癌治疗中的强硬实力。
确认客观缓解率(ORR)和疾病控制率(DCR)分别达到了73.3%和93.3%,这意味着大部分患者的肿瘤得到了不同程度的缩小或控制。中位无进展生存期(PFS)为10.9个月,中位缓解持续时间(DoR)为12.1个月,其中还有12例患者仍处于持续缓解状态。中位总生存期(OS)尚未达到,12个月和18个月的OS率分别高达92.8%和85.7%。
乳腺癌(HER2低表达):德曲妥珠单抗显著改善生存期
III期DESTINY-Breast04试验(NCT03734029)中,德曲妥珠单抗与化疗相比显著改善了转移性乳腺癌患者的总生存期(OS)和无进展生存期。373例患者接受德曲妥珠单抗治疗,184例接受化疗,结果显示:德曲妥珠单抗组中位生存期(OS)为22.9个月,化疗组为16.8个月。
HR+队列,德曲妥珠单抗组中位生存期(OS)为23.9个月,化疗组为17.6个月;
在HR−队列中,德曲妥珠单抗组中位生存期(OS)为17.1个月,化疗组为8.3个月。
而在24个月时的生存率方面,德曲妥珠单抗组也是化疗组的2倍多,分别为32.6%和11.8%。
无进展生存期(PFS)方面,德曲妥珠单抗组为8.8个月,化疗组为4.2个月。
安全性方面,两组的任何级别治疗中出现的不良事件(TEAE)发生率相似,德曲妥珠单抗组为 99.5%,化疗组为 98.3%。
目前,德曲妥珠单抗正在国内开展临床研究,有意向参加临床试验的患者请致电400-880-3716咨询康和源免疫之家。
综上可见,ADC药物具有强大的抗癌效果。
基因检测绘制“靶点地图”
但,明确靶点的存在与状态,是使用任何一款ADC药物的绝对前提。这就使得基因检测走向了临床决策的舞台。
对于蛋白过表达型靶点(如HER2、TROP2、CLDN18.2等):主要使用免疫组化(IHC)检测肿瘤组织切片中靶点蛋白的表达水平和定位,决定患者是否具备用药资格。
对于基因变异驱动型靶点(如EGFR突变、c-MET扩增等):可以借助二代测序(NGS)等技术,在基因层面检测突变、扩增或融合事件。检测报告证实了相应变异,患者才被认为是该靶向药物(包括针对此变异的ADC)的潜在获益人群。如果需要ADC药物相关抗原检测,如TROP2、CLDN18.2等,可以咨询康和源免疫之家(400-880-3716)。
结语
靶向HER2和Trop-2等ADC药物的出现,为癌症患者提供了更加精准化的治疗方法,能够显著提升治疗效果,并减少副作用。HER2、TROP2、CLDN18.2等靶点的出现照亮了这类患者的治疗之路。而基因检测制出了属于每位患者的、独一无二的“靶点地图”。随着ADC药物和靶点的爆炸式增长,未来的基因检测将朝着更全面、更整合的方向发展,为患者带来最大生存获益。
参考资料
1.https://www.cancerresearch.org/immunotherapy-by-treatment-types/targeted-antibodies
2.https://pubmed.ncbi.nlm.nih.gov/37758237/
3.https://pmc.ncbi.nlm.nih.gov/articles/PMC11416103/
4.https://www.sciencedirect.com/science/article/pii/S030573722300107X
5.https://www.sciencedirect.com/science/article/abs/pii/S0344033824006356
6.https://pmc.ncbi.nlm.nih.gov/articles/PMC10417123/
如果您在阅读本文后仍有困惑,或需要帮助,可通过康和源免疫之家医学部(400-880-3716)获取专业指导。您可以通过添加以下微信方式联系我们。
曹老师微信:17801183037
谢老师微信:15810815293
康和源免疫之家 - 癌症患者的生命希望桥梁
康和源免疫之家作为肿瘤诊疗服务平台,始终秉持“以患者为中心”的理念。在肿瘤治疗领域,患者面临着巨大的身心压力和复杂的治疗选择,康和源免疫之家深知患者的需求和困境,致力于为患者提供全方位、个性化的服务。
为了实现这一目标,该平台整合了中国、美国、欧洲、日本、韩国等全球顶尖肿瘤专家资源。这些专家拥有丰富的临床经验和先进的治疗理念,能够为患者提供专业的诊断和治疗建议。同时,平台还与知名药企、创新药物研发机构建立了深度合作关系,这使得患者能够及时了解到最新的抗癌药物和治疗技术,为治疗提供更多的选择。
国际专家会诊服务
肿瘤治疗具有复杂性和个体差异性,不同患者的病情和身体状况各不相同,因此需要个性化的治疗方案。康和源免疫之家通过国际专家会诊服务,可协调中美欧日韩等多国权威肿瘤专家,针对癌症开展跨国联合诊疗。
对于那些需要第二诊疗意见的患者来说,国际专家会诊可以提供不同的视角和建议,帮助患者更全面地了解自己的病情和治疗方案。而对于寻求海外先进治疗方案的患者,平台的专业团队能够帮助他们获取国际最新治疗指南,为患者制定更加科学、合理的治疗计划。
临床试验
作为连接患者与创新疗法的纽带,康和源免疫之家与全球领先药企及研发机构保持战略合作,持续提供涉及PD-1/L1抑制剂、CAR-T细胞治疗、ADC药物等领域的临床试验项目。这些临床试验项目代表了当前癌症治疗的最新进展和研究方向,为患者提供了接触新型抗癌药物和治疗技术的机会。
康和源免疫之家团队会根据患者的病情阶段、基因检测结果和治疗史,精准匹配适合的临床试验机会。这不仅提高了患者参与临床试验的成功率,也确保了患者能够接受最适合自己的治疗。在康和源免疫之家,每个生命都被视为值得最好的医疗选择,无论是寻求国际顶尖专家的诊疗建议,还是尝试突破性的临床试验,平台都将竭诚为患者提供专业、可靠的全方位支持。让我们携手,在抗击肿瘤的征程上走得更远。
END
如果您或您身边的人正在遭受癌症的困扰,不妨拨打咨询电话400-880-3716,登录官网 https://www.myimm.net/ 或关注微信公众号“康和源免疫之家”(头条、知乎、百度、搜狐等自媒体同名),了解更多关于临床试验和肿瘤诊疗服务的信息。
🌿 抗癌,不仅是力量的抗争,更是精准的博弈。🌿
当亲人面对癌症,我们常问:“什么治疗方案最有效?会不会产生耐药?这种病会遗传给家人吗?” 这些关乎生存与未来的关键问题,答案可能就藏在每个人的基因里。
🧬 基因检测:为患者点亮精准治疗的“导航”,为家属揭开遗传风险的“谜底”。🧬
精准打击肿瘤,避免无效治疗肿瘤千差万别,用药也需“量体裁衣”。我们的基因检测能明确揭示肿瘤的基因突变靶点,就像为导弹装上“精确制导系统”,快速匹配最可能有效的靶向药物,避免在无效的化疗中消耗宝贵的生命与希望。
评估遗传风险,为家人构筑健康防线部分癌症与遗传密切相关。通过基因检测,可以评估患者是否携带明确的遗传易感基因突变。这不仅解释了病因,更能为您的子女、兄弟姐妹等近亲提供至关重要的预警,让他们能及早干预,主动守护健康。
🔬康和源免疫之家,用基因图谱,守护您与家人的生命延续。🔬
我们提供的不仅是冰冷的报告,更是一份贯穿诊断、治疗与康复的“科学行动指南”。我们的医学专家将为您深度解读基因信息,在复杂的治疗选择中提供决策支持,并为整个家庭的健康风险管理提供专业建议。
📞 一人检测,可能惠及全家。立即拨打 400-880-3716,康和源免疫之家医学部,为您与家人的未来,提供一份科学的答案。
让科学指引方向,让选择更有力量。
扫描下方二维码,或者直接电话咨询康和源免疫之家医学部(400-880-3716),持续为您关注并分享最新消息。
免责声明:文本参考来源于网络,版权归原作者所有。
该文章仅供分享,如涉嫌侵犯您的著作权请联系我们删除,谢谢!
2025-11-24
SAN DIEGO--(BUSINESS WIRE)--Avenzo Therapeutics, Inc. (“Avenzo”), a clinical-stage biotechnology company developing next-generation oncology therapies, today announced the U.S. Food and Drug Administration (FDA) granted Fast Track designation to AVZO-103, a potential best-in-class Nectin4/TROP2 bispecific antibody-drug conjugate (BsADC).
The designation was granted for the treatment of adult patients with locally advanced or metastatic urothelial cancer who have previously received enfortumab vedotin. There are no approved antibody-drug conjugates for patients previously treated with enfortumab vedotin.
“Receiving Fast Track designation for AVZO-103 highlights the significant need for treatment options for patients with urothelial cancer who have progressed on enfortumab vedotin,” said Mohammad Hirmand, M.D., Co-founder and Chief Medical Officer of Avenzo Therapeutics. “We believe AVZO-103 has the potential to become a promising treatment option for patients and we are committed to rapidly advancing its clinical development.”
AVZO-103 is currently being studied in a Phase 1/2 first-in-human, open-label clinical study designed to assess the safety, tolerability, and preliminary clinical activity of AVZO-103 as a single agent and in combination therapy in patients with advanced solid tumors.
About Fast Track Designation
Fast Track is an FDA process designed to facilitate the development and expedite the review of drugs to treat serious conditions and fill an unmet medical need.
A drug that receives Fast Track designation is eligible for some or all of the following:
More frequent meetings with the FDA to discuss the drug's development plan and ensure collection of appropriate data needed to support drug approval;
More frequent written communication from the FDA about such things as the design of the proposed clinical trials and use of biomarkers;
Eligibility for Accelerated Approval and Priority Review, if relevant criteria are met; and
Rolling Review of a Biologic License Application or New Drug Application by the FDA
About Avenzo Therapeutics
Avenzo Therapeutics is a clinical-stage biotechnology company focused on developing next-generation oncology therapies for patients. The company’s pipeline includes potential best-in-class small molecules and antibody-drug conjugates (ADCs). Avenzo’s small molecule inhibitors, AVZO-021 and AVZO-023, are novel, highly potent and selective inhibitors of CDK2 and CDK4, respectively, which are key enzymes involved in cell cycle regulation. AVZO-021, the company’s lead drug candidate, is being studied in a Phase 1 study for the treatment of advanced solid tumors and in combinations in HR+/HER2- metastatic breast cancer. AVZO-023 is being studied in a Phase 1/2 study for the treatment of advanced solid tumors and in combinations in HR+/HER2- metastatic breast cancer. Avenzo’s first ADC drug candidate, AVZO-1418, is a potential best-in-class, EGFR/HER3 bispecific ADC that is being studied in a Phase 1/2 study for the treatment of advanced solid tumors. Avenzo’s second ADC drug candidate, AVZO-103, is a potential best-in-class, Nectin4/TROP2 bispecific ADC that is being studied in a Phase 1/2 study for the treatment of advanced solid tumors. Avenzo is headquartered in San Diego, California. For more information, visit us at www.avenzotx.com or on LinkedIn.
抗体药物偶联物快速通道临床1期加速审批
2025-11-24
🔥映恩生物 (Duality Bio) 深度解析:第三代 ADC 技术引领者的创新路径与行业价值📌一、公司概况:ADC 赛道的全球化创新标杆
映恩生物 (股票代码:9606.HK)
是一家聚焦抗体偶联药物 (ADC) 研发的临床阶段生物制药企业,由拥有 20 + 年医药投资经验 的朱忠远博士于 2019 年 7 月 创立。
作为 ADC 领域的后起之秀,公司凭借 四大原创技术平台 与 12 款差异化产品管线,
仅用 5 年时间 完成从初创到港股上市的跨越式发展,成为中国 ADC 创新药国际化的标杆企业。核心技术逻辑
ADC 药物作为生物制药领域的核心赛道,其技术核心在于 抗体靶向性、连接子稳定性、载荷毒性及偶联工艺均一性 的协同优化。映恩生物通过自主研发的技术平台,在 定点偶联技术、DAR 值(药物抗体比率)精准调控、新型载荷设计 等关键领域实现突破,旗下核心产品 DB-1303 (HER2 ADC) 已成为 全球进展第二的第三代 HER2 ADC,技术实力获国际权威验证。里程碑式 IPO(2025 年 4 月 15 日)
发行价 94.60 港元 / 股,募资总额 2.11 亿美元(行使绿鞋机制后达 2.43 亿美元),创近四年港股 18A 板块最大规模 IPO 纪录;
国际配售获 13.52 倍超额认购,上市首日股价暴涨 116.7%,收盘于 205 港元,市值突破 170 亿港元;
引入 15 家豪华基石投资者(含 BioNTech、礼来亚洲基金),共认购 6500 万美元,资本认可度拉满。全球化商业价值验证
公司累计对外授权总金额已突破 60 亿美元,构建行业领先的国际化合作网络:
与 BioNTech 达成 15 亿美元 合作,出让 DB-1303 和 DB-1311 全球权益(除大中华区);
向 GSK 授权 DB-1324,获 3000 万美元 预付款及最高 9.75 亿美元 里程碑付款;
与 Avenzo 达成 12 亿美元 双抗 ADC 交易,授权 DB-1418 全球开发权益;
四大技术平台已在全球 1000 + 患者 中完成临床验证,旁观者杀伤效应、肿瘤特异性释放 等核心指标达国际一流水平。⏳二、发展历程:技术驱动的五年高速成长(一)初创与融资奠基 (2019-2021)
2019 年 7 月:朱忠远博士在开曼注册成立,运营总部落地上海,核心团队吸纳 第一三共、罗氏 等国际药企 ADC 专家;
2020 年 4 月:完成 200 万美元 种子轮融资(6D Capital 领投),启动早期技术平台搭建;
2021 年 5 月:完成 9000 万美元 B 轮融资(礼来亚洲基金领投),投后估值约 2 亿美元,聚焦 DITAC 平台核心载荷 P1003 优化。(二)技术突破与国际化布局 (2022-2024)
2022 年:完成 1.15 亿美元 B+ 轮融资,投后估值 2.03 亿美元,四大核心技术平台(DITAC、DIBAC、DIMAC、DUPAC)全面建成;
2023 年 4 月:与 BioNTech 达成历史性合作,获 1.7 亿美元 首付款,技术平台获国际巨头认可;
2023 年底:向港交所递交 IPO 申请,同步推进 DB-1303 的 III 期临床;
2024 年 12 月:将 DB-1324 授权给 GSK,验证临床前产品全球商业价值。(三)上市与临床加速 (2025 至今)
2025 年 4 月:港股成功上市,任命 牟骅博士 为全球 CMO,前 FDA 副局长 Namandjé Bumpus 博士 加入董事会;
2025 年 5 月:行使超额配股权,额外募资 2.35 亿港元;
2025 年 7-11 月:DB-1310 获 FDA 快速通道资格、DB-1303 关键 III 期达标、DB-2304(全球首款 SLE ADC)完成首例给药,启动科创板上市(11 月与中信证券签约),"A+H" 双上市格局落地。🔬三、核心技术平台:四大原创技术构筑 ADC 创新壁垒
ADC 药物技术演进已从第一代(随机偶联、低 DAR 值、传统载荷)发展至 第三代(定点偶联、精准 DAR 值、新型载荷、双靶点设计),映恩生物四大平台均聚焦第三代核心技术,形成差异化壁垒。(一)DITAC 平台(免疫毒素抗体偶联平台)—— 拓扑异构酶 I 抑制剂的精准应用
作为核心技术平台,基于 拓扑异构酶 I 抑制剂 开发,拥有专有载荷 P1003 和 P1021,三大核心优势:定点偶联技术
:半胱氨酸定点偶联,均一性>95%,降低药代动力学波动与毒性风险;DAR 值优化
:抗体工程改造实现 DAR=8,快速毒素清除机制使血液毒性发生率降低 30% 以上;肿瘤特异性释放系统
:肽键可裂解连接子,肿瘤微环境特异性释放,结合旁观者杀伤效应覆盖异质性肿瘤。
成果:已开发 7 款临床阶段产品(含 DB-1303、DB-1311),DB-1303 III 期数据显示,ORR 较罗氏 T-DM1 提升 40%,PFS 延长 6.2 个月。(二)DIBAC 平台(双特异性抗体偶联平台)—— 双靶点协同的下一代 ADC
全球少数专注双特异性 ADC 的平台,融合 AI 靶点选择 与 双价抗体设计,三大技术突破:双靶点协同识别
:"2+2" 抗体结构,肿瘤组织富集度较单靶点 ADC 提升 2.8 倍;载荷精准递送
:延续定点偶联技术,DAR=8,搭载 P1003 形成 “双靶点 + 强效毒素” 协同;耐药性克服
:双靶点阻断逃逸通路,DB-1418 在 EGFR-TKI 耐药 NSCLC 模型中 ORR 达 58%(单靶点 ADC 仅 32%)。
成果:DB-1418 授权 Avenzo 获 5000 万美元 首付及最高 12 亿美元 里程碑;DB-1419(B7H3/PD-L1 双抗 ADC)为 全球首创,实现 “ADC 杀伤 + 免疫激活” 双重机制。(三)DIMAC 平台(免疫调节抗体偶联平台)—— ADC 向自免疾病的跨界突破
全球极少数自免疾病专属 ADC 平台,三大技术创新:免疫细胞靶向调控
:抗 BDCA2 抗体结合浆细胞样树突状细胞(pDC),精准递送免疫抑制剂,阻断 I 型干扰素通路;低毒性载荷设计
:新型免疫调节载荷,IC50 达 nM 级别,临床前模型中 SLE 患者 ANA 滴度降低 80%;长效制剂技术
:PEG 修饰延长半衰期,每 4 周给药一次,提升依从性。
成果:旗舰产品 DB-2304(全球首款 SLE ADC),2025 年 11 月完成首例给药,开创自免疾病治疗新路径。(四)DUPAC 平台(独特有效载荷抗体偶联平台)—— 耐药性解决方案
聚焦 ADC 耐药,核心突破 新型作用机制载荷开发:eIF4F 复合物靶向毒素
:创新载荷 DUP5 阻断 mRNA 翻译,对传统化疗 / ADC 耐药肿瘤有效;广谱抗肿瘤活性
:作用于非分裂期癌细胞,覆盖实体瘤(胰腺癌、胶质母细胞瘤)与血液瘤;连接子创新
:pH 敏感 + 二硫键双机制连接子,肿瘤微环境与溶酶体双重触发释放。
成果:DB-1316 等临床前产品,在 T-DM1 耐药 HER2 阳性乳腺癌模型中 ORR 达 45%。💊四、核心产品管线:临床数据驱动的价值兑现
公司管线涵盖 7 款临床阶段产品 与 5 款临床前产品,聚焦 HER2、B7H3、HER3 等高价值靶点,3 款核心产品进入临床后期。(一)DB-1303 (HER2 ADC)—— 第三代 HER2 ADC 全球竞争者
全球进展第二的第三代 HER2 ADC,采用 DITAC 平台技术,覆盖三大适应症:
乳腺癌:III 期临床纳入 628 例 患者,中位 PFS 18.7 个月(对照组 T-DM1 为 12.5 个月,HR=0.58,P<0.001),ORR 68.3%(提升 40.2%),DCR 92.1%,3 级以上不良反应降低 25%;
子宫内膜癌:全球首个 HER2 表达子宫内膜癌 ADC,III 期已完成入组;
胃癌 / 胃食管结合部癌:与 BioNTech 合作推进全球 II 期,ORR 52%,中位 PFS 9.8 个月;
上市预期:2025 年底中国申报,2026 年美国申报,有望成为 HER2 阳性肿瘤标准方案。(二)DB-1311 (B7H3 ADC)—— 多实体瘤布局的潜力品种
获 FDA 快速通道资格 + 三项孤儿药资格,聚焦多实体瘤:
CRPC(去势抵抗性前列腺癌):I/II 期纳入 52 例 患者,cORR 30.8%,DCR 90.4%,中位 DoR 8.3 个月,3 级以上中性粒细胞减少症发生率仅 11.5%;
小细胞肺癌:I 期 ORR 28.6%,中位 PFS 6.2 个月,为难治性患者提供新选择;
食管鳞癌 / 头颈部鳞癌:I/II 期 DCR 均超 85%,广谱治疗潜力显著。(三)DB-1310 (HER3 ADC)—— EGFR-TKI 耐药的解决方案
针对 EGFR 突变 NSCLC 耐药(HER3 高表达占耐药原因 15-20%):
I/II 期纳入 89 例 患者,ORR 44%,DCR 91%,中位 PFS 7.0 个月,中位 OS 18.9 个月(T790M 阳性 / 阴性患者 ORR 分别为 47%/41%);
联合治疗:与阿斯利康合作,DB-1310 + 奥希替尼用于初治患者,I 期 ORR 72%;
上市预期:获 FDA 快速通道资格,2026 年提交 NDA。(四)临床前管线:创新机制储备
DB-1324:授权 GSK,获 3000 万美元 预付款及最高 9.75 亿美元 里程碑,结直肠癌 / 胰腺癌模型 ORR 超 60%;
DB-1421:双特异性 ADC,肿瘤组织穿透性提升 3 倍,异种移植瘤 ORR 75%;
DUPAC 平台产品:DB-1316 等,T-DM1 耐药 HER2 阳性乳腺癌模型 ORR 45%,DLBCL 模型 ORR 62%。🆚五、行业对比:技术迭代与格局重塑中的核心竞争力
ADC 行业正处于 第三代技术替代第二代的关键阶段,核心竞争焦点为 技术差异化、临床优效性、国际化深度,映恩生物已确立 全球第二梯队领军地位。(一)国际巨头对标:技术路径差异化与商业化效率博弈第一三共
:第三代 ADC 开创者,DXD 平台技术成熟,Enhertu(2024 销售额 34.8 亿美元)商业化领先,但采用随机偶联技术(均一性约 70%),3 级以上不良反应发生率较映恩生物高 20%(间质性肺病发生率约 10%);辉瑞 (+Seagen)
:通过收购获得 vc-MMAE 平台,Adcetris、Padcev 已商业化(2025 上半年 Padcev 销售近 10 亿美元),但载荷毒性较强(血液毒性、神经毒性发生率高),DB-1303 血液毒性发生率较其低 35% 以上;阿斯利康
:深度绑定第一三共共享 DXD 平台,聚焦联合开发与商业化,模式偏重 “技术授权 + 合作”,而映恩生物 “自主研发 + 对外授权” 轻资产模式更灵活,60 亿美元累计授权验证技术输出能力;罗氏
:ADC 行业早期参与者,Kadcyla(T-DM1)为第二代技术,已被 Enhertu、DB-1303 全面超越,新一代产品 ARX788 临床进展滞后,技术迭代速度不足。(二)国内竞争者对比:国际化与创新深度差距荣昌生物
:国内首个 ADC 商业化企业,维迪西妥单抗(vc-MMAE 第二代平台)聚焦 HER2 单一靶点,技术平台单一,国际化授权规模(与辉瑞合作约 10 亿美元)远低于映恩生物的 60 亿美元 +;科伦博泰
:聚焦 TROP2、HER2 成熟靶点,SKB264(MMAE 载荷 + 不可切割连接子)已上市,授权默沙东获 26 亿美元,但技术路线仍以第二代为主,双抗 ADC、新型载荷布局滞后于映恩生物;乐普生物
:MRG003 为全球首个上市 EGFR ADC(vc-MMAE 平台),但授权交易规模(超 12 亿美元)有限,在自免 ADC、新型载荷等前沿领域无实质性布局。(三)核心竞争壁垒:三重护城河技术平台壁垒
:全链条自主创新,定点偶联 / 新型载荷达国际一流,70 项专利 保护至 2040 年后;管线差异化壁垒
:全球首创 SLE ADC、双抗 ADC,核心产品处于全球临床第一梯队,避免同质化竞争;商业化验证壁垒
:60 亿美元 + 国际授权,与 BioNTech/GSK 等巨头协同,加速全球落地。🎯六、投资价值与风险评估(一)核心投资亮点(技术 + 管线 + 商业化三重驱动)技术平台可扩展性
:四大平台支持多靶点 / 多适应症快速开发,DUP5 载荷、双抗技术有望引领第四代 ADC;核心产品重磅潜力
:DB-1303 瞄准 430 亿美元 + 全球市场(乳腺癌 + 子宫内膜癌 + 胃癌),DB-2304 覆盖 1000 万 + SLE 患者,均有望成为重磅炸弹;授权收入确定性
:已获 5 亿美元 首付款 + 里程碑,后续临床 / 上市节点将持续兑现现金流;估值提升机遇
:多款产品获 FDA 快速通道 / 孤儿药资格,“A+H” 上市有望享受 A 股估值溢价(港股 18A 平均 PS 15 倍 vs A 股生物药 30 倍);团队优势
:朱忠远(20 + 年投资经验)+ 邱杨(第一三共 ADC 核心开发者)+ 牟骅(国际药企高管),顾问团队含国际权威专家。(二)风险因素(四大核心风险)临床开发风险
:ADC 后期临床失败 / 延迟风险,DB-1303 与 Enhertu 头对头数据未明确,DB-2304 自免领域临床标准不成熟;竞争加剧风险
:全球在研 ADC 超 500 款,热门靶点超 30 款 竞品,国内医保谈判降价幅度达 50%-70%;合作依赖风险
:核心产品全球权益依赖 BioNTech/GSK,国内商业化依赖三生制药,合作终止 / 资源倾斜不足可能影响进度;资金消耗风险
:2024 年研发投入 8.37 亿元(占收入 40%+),III 期临床单适应症投入超 5 亿美元,资金需求持续扩大。🌍七、行业环境分析:ADC 赛道的增长逻辑与机遇(一)市场规模:精准医疗驱动千亿赛道
全球市场:2025 年预计达 160 亿美元(前三季度 121 亿美元,同比 + 25%),2022-2030 年 CAGR 20%-30%,2030 年超 600 亿美元,占生物制剂份额从 2.2% 升至 8.3%;
中国市场:2025 年规模 120-246 亿元,2030 年突破 500 亿元,CAGR 超 35%,本土企业上市 + 医保准入 + 国际化驱动增长。(二)监管与政策:创新友好型环境加速落地
全球监管:FDA 快速通道 / 突破性疗法、NMPA 优先审评 / 附条件批准,2025 年 12 款 ADC 获 FDA 快速通道(含映恩 3 款);
支付体系:中国医保谈判 “以价换量”(纳入 ADC 销售额同比 + 100%),国际市场第三代 ADC 定价溢价(Enhertu 年治疗费用 18 万美元)。(三)技术趋势:下一代 ADC 创新方向载荷迭代
:拓扑异构酶 I 抑制剂向 “高毒性 + 低脱靶” 发展,DUP5 等新型载荷应对耐药;连接子与偶联优化
:定点偶联全面替代随机偶联,智能连接子提升肿瘤特异性释放;双抗 / 多抗 ADC 爆发
:靶点组合向 “肿瘤抗原 + 免疫细胞分子” 延伸,实现 “杀伤 + 免疫激活”;适应症跨界
:从肿瘤向自免(DB-2304)、感染性疾病延伸;联合治疗深化
:ADC+PD-1/PD-L1、ADC + 靶向药,生物标志物指导个性化方案。🔮八、总结与展望(一)核心判断
映恩生物已通过 “技术创新 - 临床验证 - 国际授权 - 资本上市” 闭环,成长为中国 ADC 全球化创新标杆,核心竞争力体现在:
技术平台全球领先:四大平台覆盖第三代 ADC 核心方向,双抗 / 自免 ADC 布局引领行业;
管线差异化显著:核心产品临床数据优效,全球首创品种开辟新市场;
商业化模式验证:60 亿美元 + 国际授权,与巨头协同加速落地;
资本与人才加持:港股 + 科创板双上市,全明星团队保障执行。(二)发展预测(2025-2030)
短期(2025-2026):DB-1303 中国 / 美国申报上市,授权里程碑持续兑现,2026 年营收预计 20-30 亿元;
中期(2026-2028):DB-1303/1311/1310 相继上市,国内销售额 15-20 亿元,海外超 30 亿美元,营收突破 50 亿元;
长期(2028-2030):5-6 款产品全球上市,年营收超 100 亿元,海外收入占比 60%+,市值突破 1000 亿港元,跻身全球 ADC 第一梯队。(三)核心建议
对投资者:短期关注 DB-1303 上市进度与里程碑付款,中期跟踪商业化放量,长期把握科创板估值重估机遇,警惕临床 / 竞争风险;
对行业观察者:借鉴 “自主研发 + 国际授权” 国际化路径,关注 ADC 与 AI / 基因编辑融合,以及自免 / 感染领域临床转化;
对医药从业者:聚焦新型载荷 / 双抗 ADC / 智能连接子等源头创新,加强国际合作,推动 ADC 向精准治疗升级。
映恩生物的崛起,标志着中国 ADC 创新从 “跟跑” 进入 “并跑” 乃至 “领跑” 阶段。
在技术迭代、政策支持、市场需求驱动下,公司有望成为全球 ADC 核心玩家,为投资者带来长期回报,为患者提供更多创新治疗选择。
每日拆解一家上市公司,下期见。
△免责声明△
本文贡献分析师表达的观点并未反映出本公众号或其记者的立场,本机构分析师在所提及的任何股票中没有头寸(即做多或做空的筹码或合约),没有计划在未来72小时内发起任何头寸,也没有任何与股票有关的公司的业务关系。提及的信息仅供参考使用,可能不完整或已过时,并不构成财务,法律或投资建议。
100 项与 DB-1418 相关的药物交易
登录后查看更多信息
研发状态
10 条进展最快的记录, 后查看更多信息
登录
| 适应症 | 最高研发状态 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|---|
| 腺上皮肿瘤 | 临床2期 | 美国 | 2025-06-04 | |
| 局部晚期恶性实体瘤 | 临床2期 | 美国 | 2025-06-04 | |
| 肺癌 | 临床2期 | 美国 | 2025-06-04 | |
| 转移性实体瘤 | 临床2期 | 美国 | 2025-06-04 | |
| 实体瘤 | 临床1期 | - | - | |
| 恶性实体肿瘤 | 临床申请批准 | 中国 | 2025-11-13 |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
No Data | |||||||
登录后查看更多信息
转化医学
使用我们的转化医学数据加速您的研究。
登录
或

药物交易
使用我们的药物交易数据加速您的研究。
登录
或

核心专利
使用我们的核心专利数据促进您的研究。
登录
或

临床分析
紧跟全球注册中心的最新临床试验。
登录
或

批准
利用最新的监管批准信息加速您的研究。
登录
或

生物类似药
生物类似药在不同国家/地区的竞争态势。请注意临床1/2期并入临床2期,临床2/3期并入临床3期
登录
或

特殊审评
只需点击几下即可了解关键药物信息。
登录
或

生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用
