预约演示
MHRA approves Napp Pharmaceuticals’ Rezzayo for invasive candidiasis
2024-01-30
临床3期上市批准临床结果合格传染病产品
Preview
来源: Pharmaceutical Technology
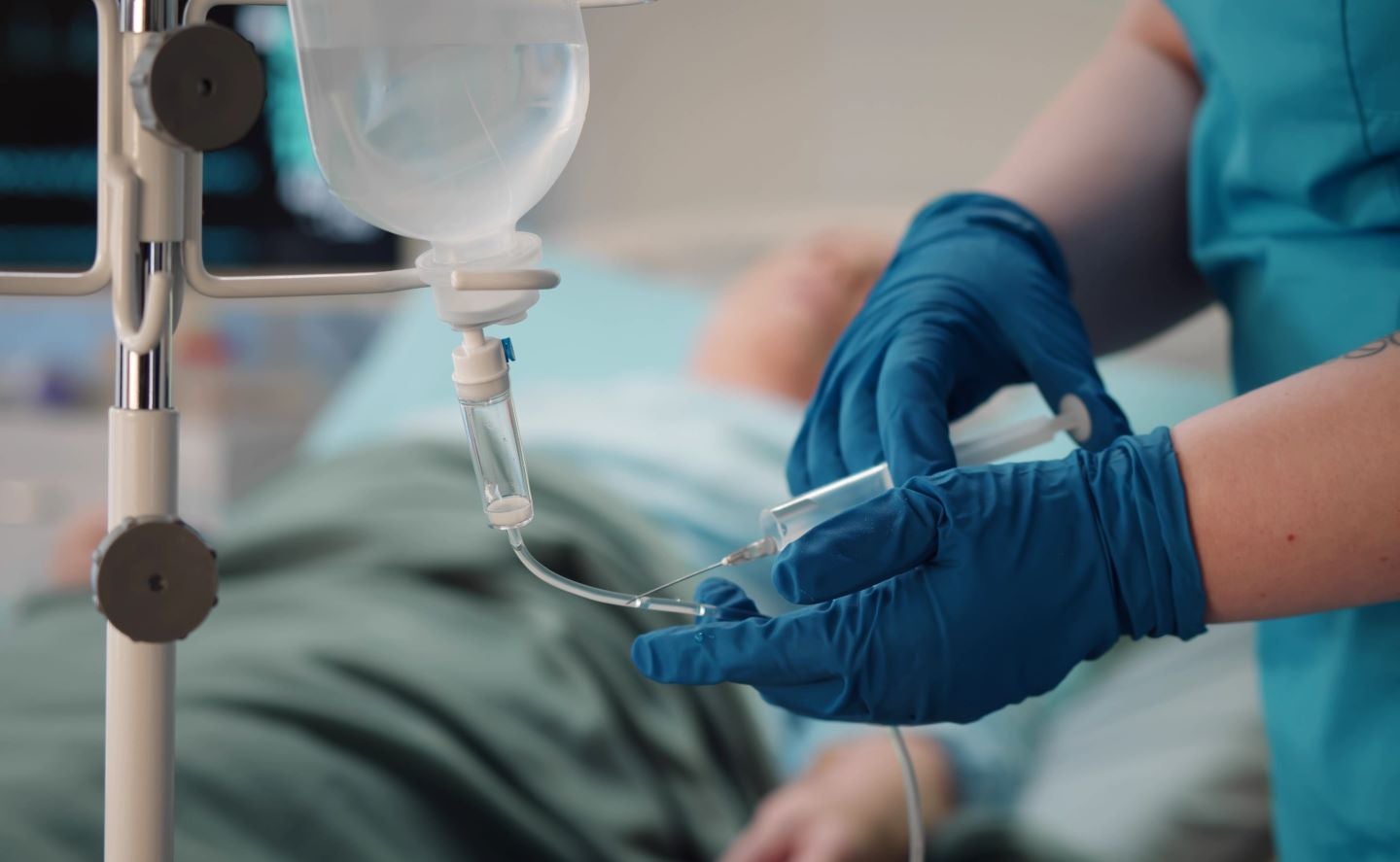
Rezzayo is indicated to be given as a weekly intravenous drip for a minimum of 14 days until Candida is no longer present in the bloodstream. Credit: TommyStockProject / Shutterstock.com.
The UK Medicines and Healthcare products Regulatory Agency (MHRA) has approved Napp Pharmaceuticals’ new antifungal treatment for invasive candidiasis, Rezzayo (rezafungin).
The fungal infection is caused by the yeast candida, which can affect areas of the body such as the blood and vital organs.
Rezzayo is given as a weekly intravenous drip for at least 14 days until candida is no longer present in the bloodstream.
Rezafungin is the active ingredient of the treatment and works by inhibiting an enzyme essential for fungal cell wall synthesis, rendering the fungal cells fragile and halting their growth.
The approval of Rezzayo is based on the results of a Phase III randomised, double-blind and controlled clinical trial.
This study involved 187 invasive candidiasis patients who were randomly given rezafungin or another antifungal medication, caspofungin, for two to four weeks.
At the 14-day mark, 55 subjects treated with rezafungin were cured, versus 57 in the caspofungin arm.
By the 30th day, 22 subjects on rezafungin had died from various causes, in contrast to 20 on caspofungin.
Hypokalaemia, diarrhoea and fever were the most common side effects of Rezzayo.
MHRA healthcare quality and access interim executive director Julian Beach said: “Keeping patients safe and enabling their access to high quality, safe and effective medical products are key priorities for us.
“We’re assured that the appropriate regulatory standards for the approval of this medicine have been met. As with all products, we will keep its safety under close review.”
更多内容,请访问原始网站
文中所述内容并不反映新药情报库及其所属公司任何意见及观点,如有版权侵扰或错误之处,请及时联系我们,我们会在24小时内配合处理。
靶点
-生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。



