预约演示
Jupiter Neurosciences, Inc. Announces Journal of Alzheimer's Disease Publication Highlighting use of JOTROL™
2022-06-14
财报
Pre-clinical proof-of-concept demonstrated in a disease model
JUPITER, Fla. and BOSTON, June 14, 2022 /PRNewswire/ --
Jupiter Neurosciences, Inc. ("Jupiter" or the "Company"), today announced the publication in the Journal of Alzheimer's Disease of "JOTROL™, a Novel Formulation of Resveratrol, Shows Beneficial Effects in the 3xTg-AD Mouse Model." This research, performed in collaboration with the University of Miami Miller School of Medicine demonstrated that a novel, oral formulation of resveratrol, JOTROL™, was able to deliver therapeutically viable levels of resveratrol in a disease mouse model of Alzheimer's disease (AD). The results showed that JOTROL™ significantly increased bioavailability over non-formulated resveratrol and that treatment resulted in AD-related gene expression changes, as well as changes in inflammatory gene and cytokine levels. JOTROL™ may be effective as a prophylaxis and/or treatment for AD through increased expression and/or activation of neuroprotective genes, suppression of pro-inflammatory genes, and regulation of central and peripheral cytokine levels. "This study demonstrated the much-improved bioavailability and activity of JOTROL™ over unformulated resveratrol in an animal model of Alzheimer's disease with extended drug exposure, stated lead author Professor Claes Wahlestedt, Associate Dean for Therapeutic Innovation, Department of Psychiatry & Behavioral Health at the University of Miami Miller School of Medicine."
Continue Reading
Preview
来源: PRNewswire
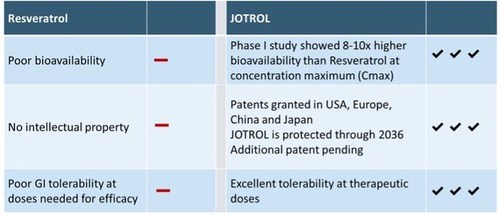
JOTROL™ Product Differentiation Comparison.
About JOTROL™
JOTROL™, the Company's unique and patented platform product, is an enhanced resveratrol formulation designed to safely deliver therapeutically relevant levels of resveratrol. In a Phase I first-in-man trial, JOTROL™ was administered in ascending doses to assess safety, tolerability, and pharmacokinetics. JOTROL™ was determined to be safe and well tolerated at all dose levels administered and achieved blood plasma target levels 8-10-fold higher than naïve resveratrol administered in historical clinical trials. The study was financed by a grant from the National Institute on Aging (NIA), entitled Safety and Pharmacokinetics of JOTROL for Alzheimer's Disease. Resveratrol can cross the blood-brain barrier and has demonstrated positive effects on oxidative stress, inflammation, and mitochondrial function in Friedreich's ataxia (FA) and Alzheimer's disease (AD) patients.
Jupiter Neurosciences, Inc. is a clinical-stage pharmaceutical company focused on treating CNS disorders and rare diseases. The Company's platform product, JOTROL™, offers potential therapeutic benefit to most central nervous system diseases such as Alzheimer's disease, ataxias, and metabolic disorders such as Lysosomal Storage Disorders and mitochondrial diseases. Human clinical trial data shows benefits in several indications and the FDA has accepted a first investigational new drug (IND) application of JOTROL™. More information may be found on the Company's website www.jupiterneurosciences.com.
Forward-Looking Statements
This press release may contain certain statements relating to future results which are forward-looking statements. It is possible that the Company's actual results and financial condition may differ, possibly materially, from the anticipated results and financial condition indicated in these forward-looking statements, depending on factors including whether results obtained in preclinical and nonclinical studies and clinical trials will be indicative of results obtained in future clinical trials; whether preliminary or interim results from a clinical trial will be indicative of the final results of the trial; the size of the potential markets for the Company's drug candidates and its ability to service those markets; and the Company's current and future capital requirements and its ability to raise additional funds to satisfy its capital needs. All forward-looking statements included in this press release are made only as of the date of this press release, and we do not undertake any obligation to publicly update or correct any forward-looking statements to reflect events or circumstances that subsequently occur or of which we hereafter become aware.
Contacts:
Investor Relations
Alison Silva, President & CBO
[email protected]
Media Relations
Alex Rosén, Chief Administrative Officer
561-406-6154
[email protected]
SOURCE Jupiter Neurosciences, Inc.
更多内容,请访问原始网站
文中所述内容并不反映新药情报库及其所属公司任何意见及观点,如有版权侵扰或错误之处,请及时联系我们,我们会在24小时内配合处理。
机构
适应症
靶点
-药物
生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。





