A Randomized, Double-blind (Sponsor-unblinded), Placebo-controlled, Single-ascending-dose Study in Healthy Volunteers and a Double-blind (Sponsor-unblinded), Placebo-controlled, Multiple-ascending-dose Study in Patients With Parkinson's Disease to Evaluate the Safety, Tolerability, and PK/PD of LY3962681
The purpose of this study is to evaluate the safety, tolerability, and PK/PD of LY3962681 in healthy volunteers and patients with Parkinson's disease.
The study will be comprised of two parts, the Single Ascending Dose (SAD) study and the Multiple Ascending Doses (MAD) study. During the SAD portion of the study, healthy volunteers will receive a single dose of LY3962681 or placebo (artificial cerebrospinal fluid (aCSF), no active drug) given into the spinal fluid. During the MAD portion of the study, patients with Parkinson's disease will receive two doses of either LY3962681 or placebo (aCSF) administered into the spinal fluid.
* The treatment period in the SAD study will be 1 day. The treatment period in the MAD study will be 2 days, 12 to 24 weeks apart.
* The follow-up period in the SAD study will be up to 52 weeks. The follow-up period in the MAD study will be up to 52 weeks post Dose 2.
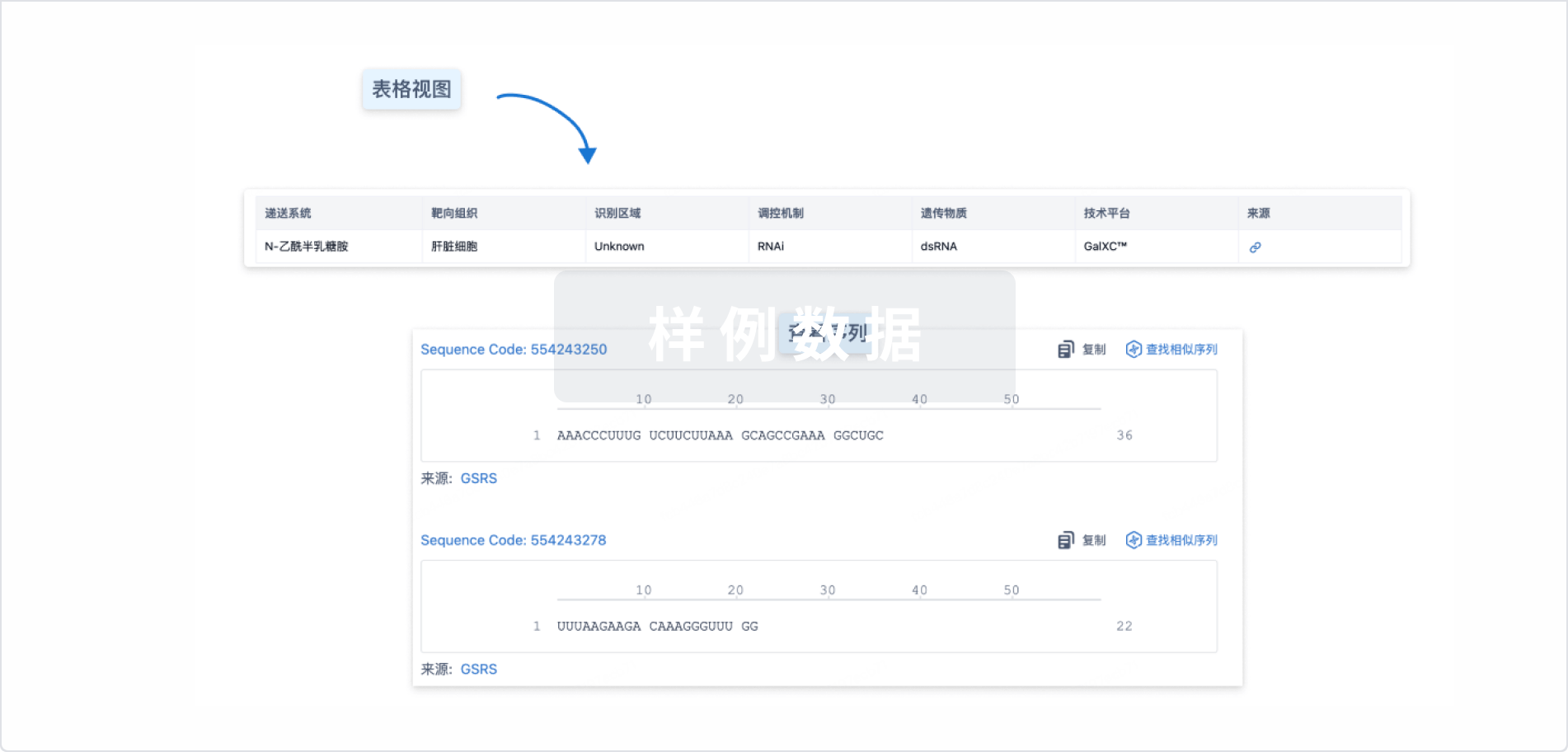
100 项与 LY-3962681 相关的临床结果
100 项与 LY-3962681 相关的转化医学
100 项与 LY-3962681 相关的专利(医药)
1
项与 LY-3962681 相关的新闻(医药)点击上方蓝字关注“钟书神经药研所”
迈克尔·J·福克斯帕金森病研究基金会(MJFF)是全球领先的帕金森病研究组织,其使命是加速开发下一代帕金森病(PD)疗法,并最终寻求治愈方法。
在一份《帕金森病重点治疗临床研发管线报告》中,来自MJFF的临床研发人员全面概述了当前帕金森病治疗领域的研发动态。
2025帕金森病整体研发管线概览
自20世纪50-60年代左旋多巴及其他主要靶向多巴胺疗法获批上市以来,工业界不断推出日益多样化的治疗手段。许多疗法仍聚焦于多巴胺能系统,以解决疾病晚期核心的运动障碍。
部分疗法则靶向其他脑内神经递质系统,有望改善帕金森病的非运动症状及其他并发症。深部脑刺激(DBS)和聚焦超声消融等外科神经调控技术的进展,也为应对运动挑战提供了更多选择。
然而,目前尚无已获批疗法证实能够延缓或阻止帕金森病的病理进程及其导致的进行性残疾,这仍是当前治疗研发管线的关键目标。
该份报告共追踪了截至2025年4月178种处于临床开发阶段的帕金森病疗法。
其中疾病修饰疗法占主导(约占51%),该类疗法旨在延缓或阻止疾病进展。其次为针对PD患者运动症状管理的疗法,占34%;针对非运动症状与并发症管理的疗法占15%。
目前处于临床阶段的疗法针对了多种广泛的PD病理生理学相关通路,反映了对PD复杂病理理解的深化。主要靶点包括:
α-突触核蛋白
LRRK2
GBA1
炎症/免疫系统
线粒体与代谢应激
多巴胺及其他神经递质系统
细胞替代疗法
在研疗法中,小分子药物仍是主流(66%),但给药方式不断创新(例如采用皮下泵形式进行给药);新型疗法如细胞疗法增长显著(12%),主要为多巴胺能细胞替代。
生物制剂包括抗体/疫苗,约占6%,肽类(6%)、基因疗法(3%)和RNA靶向疗法(2%)等新型模式不断涌现。
超过65%的临床阶段研发项目由商业公司主导,其中私营初创和新兴生物技术公司占比超过一半,显示出强大的早期创新能力。
帕金森病八大创新疗法
该报告重点跟踪了以下八大领域的创新疗法:
1. α-突触核蛋白靶向疗法
α-突触核蛋白的异常聚集是被认为是PD的核心病理标志物之一。目前在研的代表性产品包括:
抗体类疗法:如罗氏的Prasinezumab(2025年6月宣布进入III期)、Bioarctic的Exidavnemab(II期)、Lundbeck的Amlenetug(针对多系统萎缩[MSA],III期)。
主动免疫疗法(疫苗):如Vaxxinity的UB-312(I/II期)、AC Immune的ACI-7104(II期)。
降低蛋白水平的其他疗法:如礼来的siRNA药物LY-3962681(减少α-syn产生,I期)、Annovis的Buntanetap(抑制α-syn聚集,III期),Allyx的ALX-001(I期,靶向mGluR5,可抑制α-syn毒性作用)。
2. LRRK2靶向疗法
LRRK2基因突变是常见的遗传性PD风险因素,其激酶活性过高与疾病相关。目前在研的代表性产品包括:
小分子激酶抑制剂:如Denali/Biogen的DNL151(BIIB122,已进入II/III期研究阶段)、Neuron23的NEU-411(II期)。
蛋白质降解剂:如Arvinas的ARV-102(I期),可标记并清除LRRK2蛋白。
RNA靶向疗法:如Ionis的ION-859(BIIB-094,ASO,I期,但Biogen已停止开发)。
3. GBA1靶向疗法
GBA1基因突变导致葡萄糖脑苷脂酶功能受损,是PD的重要风险因素。目前在研的代表性产品包括:
小分子GCase增强剂:如Gain Therapeutics的GT-02287(I/II期)、Vanqua Bio的VQ-101(I期)。
基因疗法:如Prevail/Lilly的PR-001(II期),旨在增加功能性GCase的表达。
药物再利用(老药新用):如 Ambroxol(多个II/III期试验),初步显示可增强GCase活性。
4. 神经免疫靶向疗法
神经炎症在PD发病机制中起关键作用,通过抑制神经系统炎症可能对延缓PD疾病进展有帮助。目前在研的代表性产品包括:
NLRP3炎症小体抑制剂:NLRP3是近期研发热点之一,如Ventyx的VTX-3232(II期)、Ventus的VENT-02(II期)。
其他免疫调节抑制剂:如Biohaven的TYK2/JAK1抑制剂BHV-8000(进入II/III期)、GM-CSF(Sargramostim等,I期)、抗Galectin-3抗体TB-006(II期)。
药物再利用(老药新用):如Montelukast(II期,从哮喘药物 repurpose)。
5. 其他疾病生物学靶点疗法
GLP-1受体激动剂:多项临床研究正在进行中,但研究结果不尽一致。利司那肽(Lixisenatide)II期研究显示潜力,但艾塞那肽(Exenatide)III期研究未达主要终点。新型双靶点激动剂(如KP-405,GLP-1/GIP)也正在I期临床试验中。
靶向线粒体与能量代谢:如Mission Therapeutics的USP30抑制剂MTX-325(I期,增强线粒体自噬)、Terazosin(II期,激活PGK1改善细胞能量)。
c-Abl抑制剂:如Risvodetinib(II期,可能由新公司继续开发),但Vodobatinib等因疗效不足已停止开发。
靶向微生物组疗法:粪便微生物移植在多个II期试验中显示出对运动症状的轻度改善潜力。
6. 通用神经保护疗法
神经保护疗法旨在增强神经细胞对病理损伤的抵抗力,而非直接靶向特定病理蛋白。目前在研的代表性产品包括:
神经营养因子疗法:如Bayer/AskBio的GDNF基因疗法AB-1005(AAV2-GDNF,II期)、Herantis的CDNF模拟物HER-096(I期)。
细胞疗法:多种间充质干细胞(MSCs)疗法在研,旨在通过分泌因子提供保护(I/II期)。其他神经保护疗法包括Nurr1激动剂ATH-399A(I期)、RXR激动剂IRX-4204(I期)等。
7.细胞替代疗法
旨在通过移植多巴胺能前体细胞,替代PD患者中丢失的多巴胺神经元。近期多个早期临床试验(I/II期)报道了移植细胞的安全性和存活证据,以及初步的运动功能改善信号。
主要包括:
异体移植细胞疗法:使用胚胎干细胞或诱导多能干细胞系,如BlueRock的Bemdaneprocel(hESC来源,准备进入III期)、京都大学/Sumitomo的iPSC来源细胞(II期)。
自体移植细胞疗法:使用患者自身的iPSC,避免免疫排斥,如Aspen Neuroscience的ANPD001(II期)。
8. 靶向神经递质小分子疗法
通过影响和调节脑内神经递质水平,从而改善PD患者的运动和非运动症状。目前在研的代表性疗法包括:
针对多巴胺能疗法的优化:如新型高选择性分子或长效/缓释制剂(如AbbVie的Tavapadon,III期)、皮下输注系统(如已批准的Vyalev、Onapgo,在研的ND0612)。
非多巴胺能症状治疗:
旨在改善PD认知障碍的疗法,如Anavex的Blarcamesine(II期,靶向Sigma-1受体)。
改善PD姿势步态障碍,如IRLAB的Pirepemat(II期,靶向5-HT/去甲肾上腺素)、Atomoxetine(II期)。
改善异动症,如Celon Pharma的PDE10A抑制剂CPL500036(II期)、Neurolixis的5-HT1A激动剂Befiradol(II期)。
改善抑郁/焦虑,如Psilocybin(裸盖菇素,II期)。
基因疗法,如MeiraGTx的AAV-GAD(II期,增强GABA能信号,改善运动症状)。
更多阅读:
IRLAB宣布两篇研究摘要将在2026年AD/PD大会发表,专注帕金森病创新药物研发
【MDS 2025】非受体Abl激酶抑制剂Risvodetinib在早期帕金森病患者临床研究中显示希望
帕金森病运动波动:MDS循证医学综述更新,8种治疗方案获“有效”推荐
主要参考资料:Parkinson’s Disease Priority Therapeutic Clinical Pipeline Report ,Spring 2025 ,The Michael J. Fox Foundation for Parkinson’s Research.
声明:本文图片和内容主要来自官方和新闻网站,以及公开发表的学术会议或文献信息,如涉及版权问题,请联系删除。本文内容仅供信息分享和学术探讨之用,不构成任何形式的医学建议或健康指导。
文|神经小迷妹
医学科普 药学资讯
长按关注
100 项与 LY-3962681 相关的药物交易