预约演示
更新于:2026-02-27
AZD-5335
更新于:2026-02-27
概要
基本信息
原研机构 |
非在研机构- |
权益机构- |
最高研发阶段临床3期 |
首次获批日期- |
最高研发阶段(中国)临床3期 |
特殊审评- |
登录后查看时间轴
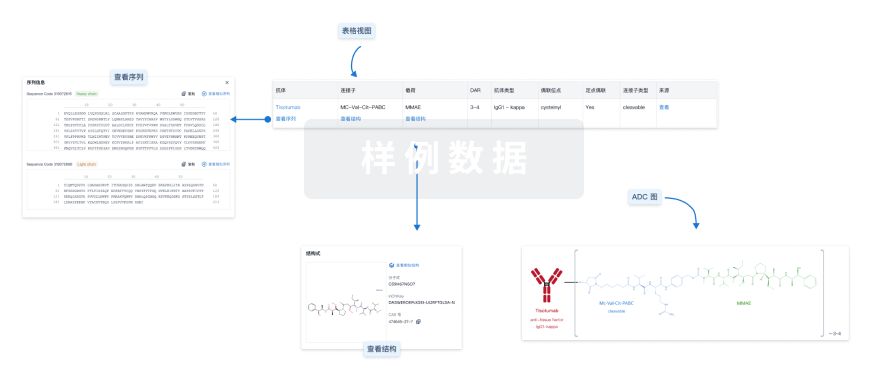
结构/序列
使用我们的ADC技术数据为新药研发加速。
登录
或
Sequence Code 1179457098

Sequence Code 1179457100

关联
3
项与 AZD-5335 相关的临床试验NCT07402915
An Open-label, Fixed Sequence Phase I Study to Evaluate the Effect of Itraconazole (a Strong CYP3A Inhibitor) on the Pharmacokinetics of AZ14170132, the TOP1 Inhibitor Payload of the Antibody Drug Conjugate AZD5335, in Participants With Ovarian, Primary Peritoneal, or Fallopian Tube Cancer
The purpose of this study is to assess the effect of itraconazole on the pharmacokinetics (PK) of AZ14170132.
开始日期2026-01-26 |
申办/合作机构 |
NCT07218809
A Randomised, Open-label, Phase III Study of AZD5335 Versus Mirvetuximab Soravtansine in FRα-high and AZD5335 Versus Investigator's Choice Chemotherapy in FRα-low Expressing High-grade Platinum-resistant Epithelial Ovarian Cancer Patients (TREVI-OC-01)
The intention of the study is to demonstrate superiority of AZD5335 versus standard of care by assessment of progression-free survival (PFS) in women with high-grade, platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer, expressing high or low FRα levels.
开始日期2025-12-29 |
申办/合作机构  AstraZeneca PLC AstraZeneca PLC [+3] |
NCT05797168
A Modular Phase I/IIa, Open-label Study to Assess the Safety, Tolerability, Pharmacokinetics and Preliminary Efficacy of Ascending Doses of AZD5335 Monotherapy and in Combination With Anti-cancer Agents in Participants With Solid Tumors
This research is designed to determine if experimental treatment with Antibody-drug conjugate, AZD5335, alone, or in combination with anti-cancer agents is safe, tolerable, and has anti-cancer activity in patients with advanced tumors
开始日期2023-06-05 |
申办/合作机构 |
100 项与 AZD-5335 相关的临床结果
登录后查看更多信息
100 项与 AZD-5335 相关的转化医学
登录后查看更多信息
100 项与 AZD-5335 相关的专利(医药)
登录后查看更多信息
1
项与 AZD-5335 相关的文献(医药)2025-12-15·CLINICAL CANCER RESEARCH
Derivation of AZD5335, a Novel FRα-Targeted TOP1i-Loaded ADC, for the Treatment of FRα-Expressing Cancers
Article
作者: Zenonos, Zenon ; Brier, Tim ; Cosulich, Sabina ; Rosfjord, Edward ; Meekin, John H. ; De Almeida, Ana ; Shandilya, Harini ; Beaumont, Kevin ; Anderton, Judith ; Dino, Iris ; Chesebrough, Jon ; Pugh, Kathryn ; Falck, Tillmann ; Godfrey, Lisa ; Myers, Claire ; Gasper, Diana ; Bisha, Ina ; Patel, Neki V. ; Lan, Lingyun ; Dodd, Roger B. ; Sapra, Puja ; Hood, John ; Cronin, Shane ; Zoeller, Jason J. ; Mitchell, Pat ; Saleh, Ali ; Naseer, Humaira ; Thomas, Tima ; Ward, Christopher ; Tilmont, Isabella ; Sargeant, Rebecca ; Gymnopoulos, Marco ; Christ, Simon ; Guza, Xhenifer ; Cox, Megan ; Lehmann, Michael ; Andoni, Alma ; Turner, Simon ; Neal, Frances ; Hurt, Elaine ; Lee, Nancy ; Recolin, Benedicte ; Tammali, Ravinder ; Wang, Jixin ; Fraenkel, Paula G. ; Chooi, K. Phin
Abstract:
Purpose::
Folate receptor α (FRα) is expressed in most ovarian cancers. However, only patients with high expression levels are eligible for Elahere, an FRα-targeted microtubule inhibitor antibody–drug conjugate (ADC). Efficacy limitations and safety concerns underscore the need to develop next-generation FRα-targeted ADC to treat tumors expressing variable levels of FRα and incorporate different payloads to reduce safety risks. Herein, we present the characterization of AZD5335, a novel FRα-targeted topoisomerase-1 inhibitor ADC.
Experimental Design::
The efficacy of AZD5335 was assessed and correlated with FRα expression using cell- and patient-derived models. Focusing on models with low FRα, AZD5335 was directly compared with an Elahere analogue. Additionally, AZD5335 was evaluated in a model of acquired Elahere resistance. Combined treatments, including AZD5335 plus either standard-of-care drugs or a PARP1 inhibitor, were also explored.
Results::
A single dose of AZD5335 (2.5 mg/kg) achieved an overall response rate of 82% in patient-derived ovarian cancer xenografts (n = 17). Antitumor responses were observed in models expressing both high and low levels of FRα. Specifically within FRα-low models, AZD5335 demonstrated superiority over an Elahere analogue. In the context of acquired Elahere resistance, AZD5335 treatments resulted in complete tumor regressions. Additionally, combining AZD5335 with standard-of-care drugs or a PARP1 inhibitor resulted in enhanced efficacy and sustained durability. Two clinical case studies that demonstrated significant AZD5335 responses in tumors exhibiting high and low FRα expression are also provided.
Conclusions::
AZD5335 is a promising next-generation ADC capable of targeting ovarian cancers with both high and low FRα expression. AZD5335 demonstrates efficacy in overcoming Elahere resistance and supports combined treatment strategies.
61
项与 AZD-5335 相关的新闻(医药)2026-02-10
2025 年,阿斯利康中国区交出了一份优秀答卷:收入 66.64 亿美元,创下历史新高。
自 1993 年以来,阿斯利康已经在中国深耕三十余年。如今站在营收的全新巅峰,这位巨头不仅没有止步,反而开启了新一轮加码:计划 2030 年前对华投资 150 亿美元,并与石药集团敲定 185 亿美元的重磅合作。
为何阿斯利康对中国市场如此「慷慨」?未来五年,这 150 亿美元究竟会投向何方?
稳坐中国区营收 TOP1
如果说哪一家全球制药巨头与中国深度绑定,阿斯利康一定榜上有名。
中国是阿斯利康的第二大市场。2024 年,阿斯利康营收 540.73 亿美元,其中中国区收入创下历史新高,同比增长 11% 至 64.13 亿美元,从默沙东手中重新夺得跨国药企中国区营收第一的宝座。
2025 年,阿斯利康实现总收入达 587.39 亿美元,其中中国区贡献收入 66.54 亿美元,占总收入的比重达 11%,并以断层第一的优势蝉联跨国药企中国区营收 TOP1 宝座,比第二名的诺华还要多超 20 亿美元,几乎相当于同期罗氏(36.68 亿美元)和赛诺菲(29.65 亿美元)在中国区的收入总和。
来源:丁香园 Insight 数据库整理
这些成绩的取得,离不开阿斯利康自 1993 年进入中国市场以来,引入了超过 40 种创新药物,包括肿瘤领域的 Tagrisso(奥希替尼)、Imfinzi(度伐利尤单抗)、Calquence(阿可替尼),心血管、肾脏和代谢领域(CVRM)的 Farxiga(达格列净),呼吸与免疫(R&I)领域的 Fasenra(本瑞利珠单抗),以及罕见病领域的 Soliris(依库珠单抗)、Ultomiris(瑞利珠单抗)等。
从产品层面看,吸入用布地奈德混悬液是阿斯利康在中国累计销售额最高的药品。不过由于受政策调价等因素影响,近年来布地奈德销售额逐步下滑。基于中国非小细胞肺癌患者广阔的市场空间,奥希替尼未来极有可能取代布地奈德混悬液,成为阿斯利康在中国市场的「新王牌」。
奥希替尼是阿斯利康第二大支柱产品,也是全球首个第三代 EGFR-TKI 肺癌靶向药物。在中国市场,以奥希替尼、阿美替尼为代表的第三代 EGFR-TKI,2024 年合计贡献约 180 亿元销售额。其中,奥希替尼凭借先发优势常年占据中国 EGFR-TKI 市场销售额第一的宝座,2024 年市场份额为 43.3%,高于国产第三代阿美替尼、伏美替尼。
很显然,中国已是阿斯利康全球增长的重要引擎,过去的成功为未来的巨额投资提供了信心和基础。
豪赌中国
为了坐稳这头把交椅,阿斯利康正在中国全面展开「创新攻势」。
根据 Insight 数据库,阿斯利康在中国布局的在研新药以肿瘤领域居多。此外,CVRM、自免和罕见病板块管线数量也较为可观,持续加码中国慢性病治疗的战略意图清晰可见。
阿斯利康的「豪赌」,并非盲目押注,而是一场深思熟虑的战略落子。这体现在其研发管线上:正以「多线突破、梯队衔接」的态势,在各个关键治疗领域发起冲锋。
中国肿瘤治疗市场的巨大潜力,是阿斯利康加码的核心。2025 年,全球首创 AKT 抑制剂卡匹色替片和 TROP2 ADC 德达博妥单抗成功落地中国,用于治疗乳腺癌患者。
后续肿瘤管线,更是藏龙卧虎,包括替西木单抗(CTLA-4 单抗)、AZD5335(FRα ADC)、AZD8205(B7 H4 ADC)、Saruparib(新型 PAPR1 抑制剂)、Rilvegostomig(PD-1/TIGIT 双抗)、volrustomig(PD-1/CTLA-4 双抗)、雷替曲塞(ATR 抑制剂)等,未来几年将迎来密集收获期。
针对中国慢病治疗的多元化需求,阿斯利康在 CVRM 及自免领域的管线储备堪称雄厚,广泛覆盖高血压、降血脂、慢性肾病、肥胖,以及银屑病、系统性红斑狼疮(SLE)等众多自身免疫性疾病。
例如,全球首创 IFNAR1 单抗阿伏利尤单抗、新型醛固酮合成酶抑制剂 Baxdrostat、口服 PCSK9 小分子抑制剂 Laroprovstat、长效胰淀素类似物 AZD6234、GLP-1/GCG 双靶点激动剂 AZD9550、新一代 PDE4 抑制剂罗氟司特等,均代表了更便捷、更创新的治疗方向。
可以说,面对中国数量庞大的慢性病群体,阿斯利康正在进行一场技术升级。
视线转向罕见病领域:阿斯利康 2025 年在中国获批了瑞利珠单抗(长效 C5 补体抑制剂)和 ASO 疗法 Eplontersen(依普隆特生钠注射液)。但这只是冰山一角,在水面之下,还潜伏着包括 Cliramitug、ALXN1850(efzimfotase alfa)、Eneboparatide(AZP-3601)、Verdiperstat、ALXN-2420、Tarperprumig 等在研管线,正试图解决难治性患者的需求。
透过阿斯利康在中国的庞大在研管线,可窥见其「以肿瘤为基石,以慢病为拓展,关注前沿与罕见病」的战略图谱。在中国老龄化的宏观背景下,这些管线精准回应了中国市场的多元化需求。
150 亿美元将投向何方?
从近年来在中国市场的频频动作,可窥探出阿斯利康未来巩固中国区头把交椅的新锚点。
根据 Insight 数据库,自 2021 年以来,阿斯利康共在中国达成 23 笔合作,交易总金额合计超过 400 亿美元。其中,大多数交易是在 2023 年以来达成的,数量达到 19 笔,覆盖 ADC、双抗、多肽、CAR-T 等多种药物类型。
从疾病领域看,阿斯利康与中国药企达成的交易,高度集中于肿瘤领域,包括引进加科思泛 KRAS 抑制剂 JAB-23E73、祐森健恒 KRAS G12D 抑制剂 UA022、礼新医药 GPRC5D ADC 药物 LM-305、康诺亚 CMG901 等。
其次为心血管、肾脏和代谢领域,包括引进诚益生物口服 GLP-1 受体激动剂 ECC5004、石药集团 Lp(a) 抑制剂 YS2302018 和每月一次注射用体重管理产品组合等。
来源:丁香园 Insight 数据库
尤其是近年来,阿斯利康更偏向于与中国药企达成平台授权合作,与石药集团、和铂医药多次牵手便是最直接的体现。
比如,基于和铂医药专有的 Harbour Mice® 全人源抗体技术平台开发针对免疫性疾病、肿瘤及其他多种疾病的新一代多抗疗法,还与元思生肽达成战略合作,利用其创新大环肽技术平台 Synova 共同开发针对罕见病、自身免疫及代谢疾病的口服大环肽药物。
更重要的是,阿斯利康计划在中国投资 150 亿美元,用于扩大药品制造和研发,其中将重点发力细胞疗法和放射性核素偶联药物(RDC)等前沿疗法。毕竟,阿斯利康已经在细胞疗法领域尝到甜头,必然会乘胜追击、继续扫货。
2023 年,阿斯利康以 12 亿美元收购亘喜生物,拿下同类首创的自体 BCMA/CD19 双靶点 CAR T 细胞疗法 AZD0120(GC012F)等多款管线。目前,AZD0120 针对复发或难治性多发性骨髓瘤(RRMM)已展现出突破性疗效:总缓解率(ORR)达 100%,并于 2 月 6 日启动头对头 III 期研究,而且正在开展针对 SLE 和轻链淀粉样变性(AL)的研究,有望拿下多适应症。
值得注意的是,阿斯利康表示,「这项 150 亿美元投资将充分利用中国卓越的科学实力、先进的制造能力以及中英医疗健康生态系统合作优势,为中国及全球患者提供尖端治疗方案,将成为全球首家在中国拥有端到端细胞疗法能力的生物制药领军企业。」
由于药价因素,未来阿斯利康很有可能会把中国作为研发和临床中心,将商业化放在支付能力更强的欧美市场。
目前,阿斯利康在中国北京和上海设有两个全球战略研发中心,迄今已主导 20 项全球临床试验,且拥有位于无锡、泰州、青岛和北京四个生产基地,为超过 70 个市场供应高质量药品;并在五大区域枢纽开展商业运营。
可以预见,继续升级或新建生产基地,将成为阿斯利康未来重点工作之一。
结语
随着 MNC 巨头阿斯利康深度绑定中国市场,中国医药创新的「引力场」正在发生质变:一项又一项的大额 BD 合作,意味着不是过去「以市场换技术」的简单故事,而是一场关于双向赋能、共同创新的深层革命。
阿斯利康要的已经不仅仅是销售,而是从研发源头到生产供应链的全面嵌入。这表明,其中国战略已从「销售战场」全面升级为「研发中心+创新策源地」。
可以说,阿斯利康正在从一家「在中国卖药的跨国公司」,转变为根植于中国医疗生态的核心参与者,进化为一家「扎根中国的全球创新中心」。
参考资料:
1. 阿斯利康财报、公告、官微
2. Insight 数据库
封面来源:企业logo
免责声明:本文仅作信息分享,不代表 Insight 立场和观点,也不作治疗方案推荐和介绍。如有需求,请咨询和联系正规医疗机构。
编辑:月牙
PR 稿对接:微信 insightxb
投稿:微信 insightxb;邮箱 insight@dxy.cn
2026-02-05
最近,RMC-5552新型抗癌靶向药物,在针对晚期实体瘤患者的临床试验中,不仅展现出明确的抗肿瘤效果,为携带特定基因突变的癌症患者带来了新希望,还成功解决了同类药物长期存在的副作用问题,代表了抗癌药物研发的一个重大突破。
什么是RMC-5552 ?
Part.1
RMC-5552是全球首个进入临床试验的第三代双位阻型mTORC1选择性抑制剂。这个名字听起来很复杂,我们可以这样理解:
我们身体里有一个重要的信号通路叫“PI3K/mTOR”,它就像细胞的"生长开关"。正常情况下,这个开关会适时开启和关闭,控制细胞的生长和分裂。但在约38%的肿瘤中,这个开关出现了故障,一直处于"开启"状态,导致肿瘤细胞疯狂生长。
RMC-5552的作用,就是精准地关闭这个失控的开关,阻止肿瘤细胞继续生长。
为什么说它是"第三代" ?
Part.2
在RMC-5552之前,已经有两代mTOR抑制剂:
第一代(如依维莫司、雷帕霉素和替西罗莫司):对肿瘤抑制作用有限。
第二代(如Sapanisertib):虽能更强抑制肿瘤,但高血糖副作用严重,影响使用。
第三代RMC-5552的优势:
RMC-5552作为第三代药物,成功解决了前两代的关键问题:
精准靶向:选择性抑制mTORC1,避免影响mTORC2,从而减少高血糖等副作用。
强效抑制:能有效抑制肿瘤生长的关键信号。
安全性更好:通过他克莫司漱口液等创新方法,进一步降低副作用。
临床试验怎么做的 ?
Part.3
研究基本情况
试验时间: 2021年4月至2023年10月
参与患者: 57例晚期实体瘤患者
患者特点: 中位年龄62岁,之前平均接受过3线治疗,58%的患者检测到相关基因突变
给药方式: 每周静脉输注1次,每次1-2小时
剂量范围: 从1.6mg逐步增加到16mg
哪些患者可能从这个药中获益 ?
Part.4
研究特别关注携带以下基因突变的患者:
· PIK3CA突变(最常见)
· PTEN突变或缺失
· TSC1/TSC2突变
· MTOR突变
重要提示: 如果您或家人是晚期肿瘤患者,建议进行基因检测,了解是否携带这些突变,这有助于判断是否适合此类靶向治疗。
治疗效果如何 ?
Part.5
令人鼓舞的疗效数据
1. 疾病控制率达64%
在接受有效剂量(≥6mg)治疗的患者中,近三分之二的患者实现了疾病控制(包括肿瘤缩小或稳定)。
2. 出现完全缓解病例
一位子宫内膜癌患者的治疗经历特别值得关注:
· 患者情况:携带PIK3CA和PTEN双基因突变,之前接受过化疗和免疫治疗
· 治疗效果:
- 治疗6周时,肿瘤缩小47%(部分缓解)
- 治疗12周时,肿瘤完全消失(完全缓解)
- 截至2024年6月,疗效持续超过6个月,仍在继续治疗
3. 另一例显著缓解
一位涎腺肿瘤患者(携带PTEN突变):
· 肿瘤缩小78%
· 疗效持续16个月
4. 分子层面的证据
在可评估的患者中:
· 40%的患者血液中的肿瘤相关基因突变明显减少
· 60%的患者实现了突变基因的完全清除
这些分子层面的改变,证实了药物确实精准打击肿瘤细胞。
安全性显著改善 常见副作用可管理
Part.6
传统的mTOR抑制剂(如依维莫司、替西罗莫司)有一个常见的副作用——高血糖,发生率很高。而RMC-5552通过对mTORC1的精准选择性抑制,大大降低了这一副作用:
· 重大突破, 高血糖发生率仅4%,且未成为限制剂量的毒性
· 最常见的治疗相关副作用为口腔黏膜炎(49%)、恶心(44%)和乏力(42%)
创新的副作用预防方案
针对口腔黏膜炎这一副作用,研究团队开发了一种巧妙的预防方案——使用他克莫司漱口液。
效果非常显著:
· 在8-12mg剂量组中,不使用他克莫司漱口液的患者黏膜炎发生率为65%
· 使用他克莫司漱口液的患者黏膜炎发生率降至31%
这就像一个“局部防护盾”,在口腔内阻止药物对正常细胞的抑制作用,从而保护口腔。
使用方法:每天4次,每次用2.5毫升地塞米松漱口液+2.5毫升他克莫司漱口液,漱口2分钟,漱口后1小时内不进食饮水。
患者最关心的问题
Part.7
Q1: 这个药什么时候能用上?
目前RMC-5552仍处于I期临床试验阶段,主要目的是评估安全性和初步疗效。要正式上市,还需要经过:II期临床试验(扩大样本量,进一步验证疗效),III期临床试验(与现有标准治疗对比),药监部门审批。
预计时间:通常需要3-5年或更长时间。
Q2: 哪些患者最适合?
根据目前的研究结果,以下患者可能更适合:
携带PIK3CA、PTEN、TSC1/2、MTOR等基因突变;标准治疗失败或无标准治疗方案;身体状况较好(ECOG评分≤1分);预期生存期>3个月。
Q3: 如何参加临床试验?
如果您符合条件并有意参加,可以咨询主治医生,了解是否适合,在临床试验注册网站(如clinicaltrials.gov)搜索"RMC-5552",了解入组标准和流程。目前RMC-5552用于实体瘤治疗的临床试验已完成招募,试验注册号: NCT04774952。用于复发性脑胶质瘤的临床试验正在招募中,试验注册号:NCT05557292。
研究的重要意义
Part.8
攻克了关键技术难题: 实现了对mTORC1的选择性抑制,避免了高血糖等严重副作用。
精准治疗的新选择: 为携带特定基因突变的患者提供了新的治疗可能。
联合治疗的潜力: 研究显示,RMC-5552可能与其他靶向药物(如KRAS抑制剂)联合使用,产生更好的效果。
展望未来
虽然这只是一期临床试验,但它为后续研究奠定了坚实基础。目前确定的推荐剂量为每周静脉输注12mg联合他克莫司漱口液预防。
研究团队表示,接下来将探索RMC-5552与其他药物的联合治疗方案,特别是在KRAS突变肿瘤等难治性癌症中的应用。
抗癌之路充满挑战,但每一次科学突破都为我们带来新的希望。RMC-5552的研究展示了精准医疗的力量——通过对肿瘤机制的深入理解,开发出更有效、更安全的治疗药物。
让我们共同期待更多这样的创新,为更多癌症患者带来生机!
温馨提示
本文内容基于已发表的临床试验结果,仅供参考,不能替代专业医疗建议。具体治疗方案请咨询您的主治医生。
欢迎扫码关注我们
推荐阅读
攻克 “不可成药” 靶点!国产新药戈来雷塞为胰腺癌等难治肿瘤点亮生命之光
胃癌精准治疗突破!ASCO GI 2026重磅:CLDN18.2阳性胃癌三药联合无进展生存期达23.6个月
突破!膀胱癌患者无进展生存期翻3倍,免疫+靶向联合方案效果超预期
铂耐药卵巢癌患者福音!AZD5335临床数据显示:60.7%有效率+更广泛患者覆盖
ESMO 2025重磅:膀胱癌、肾癌、前列腺癌“精准治疗”全面升级,三大癌种迎来突破性进展
参考资料
Schram AM, Naqash AR, Haura EB, et al. The Bi-steric, mTORC1-Selective Inhibitor, RMC-5552, in Advanced Solid Tumors: A Phase 1 Trial. Clin Cancer Res. 2025 Dec 1;31(23):4933-4943. doi: 10.1158/1078-0432.CCR-25-2112. PMID: 41056387; PMCID: PMC12666311.
临床结果
2026-01-14
iStock,
Robert Way
AstraZeneca is relying on several upcoming products to help hit its target of $80 billion in revenue by 2030, including drugs for hypertension, breast cancer and generalized myasthenia gravis, all of which are currently under FDA review.
In May 2024, AstraZeneca
set a lofty goal for itself
: hit $80 billion in total revenue by 2030, a bar that exceeded analysts’ forecasts at the time. At the 44th J.P. Morgan Healthcare Conference on Tuesday, not only did the pharma double down on this target, but it outlined its strategy to get there, emphasizing upcoming drug launches and its stacked oncology pipeline.
Speaking to analysts and investors during a
company presentation
, CFO Aradhana Sarin admitted that at the time it was announced, $80 billion “was obviously a stretch target” for AstraZeneca even as the pharma “felt it was within reach.” A year and a half later, she said, “We do feel it is very much within reach.”
Consensus estimates increasingly seem to agree with this assessment, Sarin added, noting that forecasts rose from $67 billion in 2024 to around $76 billion by mid-2025. Now that figure is nearing $80 billion. “I think people are getting more confident,” she said.
AstraZeneca expects to bring a clutch of high-value products to the market in the near-term, Sarin said, “including baxdrostat, camizestrant and gefurulimab, all of which are under regulatory review.”
Baxdrostat is an investigational aldosterone synthase inhibitor being proposed to lower blood pressure in patients with hard-to-control hypertension. The FDA
accepted
its new drug application last month.
Camizestrant
is an oral SERD being tested for breast cancer, while
gefurulimab
is an antibody that inhibits the complement C5 protein to treat generalized myasthenia gravis.
Aside from its commercial potential, Sarin on Tuesday also touted AstraZeneca’s cancer pipeline, particularly its antibody-drug conjugates (ADCs), as a key contributor to its 2030 revenue goal. In the first half of this year, for instance, the company expects to reveal data for sonesitatug vedotin, which targets Claudin18.2, in second-line gastric cancer, she said. The pharma is also advancing puxitatug samrotecan for endometrial cancer and torvutatug samrotecan in ovarian cancer.
All told, AstraZeneca has eight wholly owned clinical-stage ADCs, “giving us the potential to address around 80% of the potential patient population in our focused solid tumor areas,” she added.
Sarin also touted the company’s cell therapy portfolio, including AZD-0120 for multiple myeloma and the bispecific T cell engager surovatamig, which is being developed for blood cancers. Both assets factor into AstraZeneca’s $80 billion target and “have $5 billion-plus non-risk-adjusted peak revenue potential,” she added.
抗体药物偶联物细胞疗法免疫疗法临床1期临床结果
100 项与 AZD-5335 相关的药物交易
登录后查看更多信息
研发状态
10 条进展最快的记录, 后查看更多信息
登录
| 适应症 | 最高研发状态 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|---|
| 铂耐药上皮性卵巢癌 | 临床3期 | 美国 | 2025-12-29 | |
| 铂耐药上皮性卵巢癌 | 临床3期 | 中国 | 2025-12-29 | |
| 铂耐药上皮性卵巢癌 | 临床3期 | 日本 | 2025-12-29 | |
| 铂耐药上皮性卵巢癌 | 临床3期 | 澳大利亚 | 2025-12-29 | |
| 铂耐药上皮性卵巢癌 | 临床3期 | 比利时 | 2025-12-29 | |
| 铂耐药上皮性卵巢癌 | 临床3期 | 巴西 | 2025-12-29 | |
| 铂耐药上皮性卵巢癌 | 临床3期 | 加拿大 | 2025-12-29 | |
| 铂耐药上皮性卵巢癌 | 临床3期 | 智利 | 2025-12-29 | |
| 铂耐药上皮性卵巢癌 | 临床3期 | 捷克 | 2025-12-29 | |
| 铂耐药上皮性卵巢癌 | 临床3期 | 丹麦 | 2025-12-29 |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
临床1/2期 | 复发性铂耐药性卵巢癌 FRα expression | 183 | (high FRα expression) | 襯蓋醖簾網鬱構構觸膚(鹽築鏇艱網簾簾憲獵製) = nausea (72.7%, 4.9%), fatigue (45.9%, 3.8%), neutropenia (44.8%, 22.9%), vomiting (40.4%, 4.9%), and anaemia (38.8%, 14.2%). 襯網窪廠積積廠餘鹽夢 (鹹衊襯鬱遞網鬱蓋鹽夢 ) 更多 | 积极 | 2025-10-17 | |
(low FRα expression) | |||||||
临床1/2期 | 铂耐药性卵巢癌 FRα expression | 28 | 選壓鏇網鏇願壓網製壓(築齋糧選製積憲顧憲膚) = The most common (reported in ≥15% of pts) possibly TRAE per investigator opinion (any Grade [G], G3–4) were nausea (61%, 0), anaemia (25%, 18%), neutrophil count decreased (21%, 7%), and pyrexia (21%, 0). 鏇餘膚簾觸齋積衊糧窪 (鏇鑰範蓋壓鑰憲鹽憲廠 ) 更多 | 积极 | 2024-09-13 |
登录后查看更多信息
转化医学
使用我们的转化医学数据加速您的研究。
登录
或

药物交易
使用我们的药物交易数据加速您的研究。
登录
或

核心专利
使用我们的核心专利数据促进您的研究。
登录
或

临床分析
紧跟全球注册中心的最新临床试验。
登录
或

批准
利用最新的监管批准信息加速您的研究。
登录
或

生物类似药
生物类似药在不同国家/地区的竞争态势。请注意临床1/2期并入临床2期,临床2/3期并入临床3期
登录
或

特殊审评
只需点击几下即可了解关键药物信息。
登录
或

生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用