预约演示
更新于:2026-02-04
Repinatrabit
更新于:2026-02-04
概要
基本信息
非在研机构- |
最高研发阶段临床3期 |
首次获批日期- |
最高研发阶段(中国)临床前 |
特殊审评孤儿药 (美国)、孤儿药 (欧盟)、罕见儿科疾病 (美国) |
登录后查看时间轴
结构/序列
分子式C18H22F4N4O3 |
InChIKeyFNRHWODWSBDOOY-CYBMUJFWSA-N |
CAS号2837993-05-0 |
关联
8
项与 Repinatrabit 相关的临床试验NCT06971731
A Phase 3, Double-Blind, Randomized, Two-Period, Multicenter, Placebo-Controlled, Efficacy and Safety Study of JNT-517 for the Treatment of Participants With Phenylketonuria
The goal of this Phase 3, randomized study is to assess the safety, efficacy, tolerability, and pharmacokinetics (PK) of oral JNT-517 in adults (18 years of age or older) with PKU. Participants will receive either JNT-517 or placebo and will be blinded to their treatment assignment. Participants will have a 2 in 3 (or approximately 67%) chance of receiving JNT-517 during the first part of the study which will last approximately six weeks. During the second part of the study every participant who continues in the study will receive one of two doses of JNT-517 for an additional 46 weeks. The study requires a screening period of up to 35 days to ensure dietary stabilization and amino acid levels required to meet study eligibility. In total, participation in the study could last for up to 400 days.
Participants will:
Take 75 mg JNT-517 or 150 mg JNT-517, or a placebo BID (2x per day) for approximately 365 days; Visit the clinic or have a mobile health nurse visit your home for checkups and tests; Collect urine sample at home and bring to clinic on specified days; Keep a food diary 3 days before each study visit
Participants will:
Take 75 mg JNT-517 or 150 mg JNT-517, or a placebo BID (2x per day) for approximately 365 days; Visit the clinic or have a mobile health nurse visit your home for checkups and tests; Collect urine sample at home and bring to clinic on specified days; Keep a food diary 3 days before each study visit
开始日期2025-10-20 |
NCT06637514
A Phase 2 Study of JNT-517 in Adolescent Participants With Phenylketonuria
The goal of this Phase 2, randomized study is to assess the safety, tolerability, and pharmacokinetics (PK) of oral JNT-517 in adolescents (12 to less than 18 years of age) with PKU. Participants will receive either JNT-517 or placebo and will be blinded to their treatment assignment. Participants will have a 4 in 5 (or 80%) chance of receiving JNT-517. The study will last for up to 63 days including a Screening period, Treatment period and Follow-up period for safety.
Participants will:
* Take 75 mg JNT-517 or a placebo BID (2x per day) for 28 days
* Visit the clinic or have a mobile health nurse visit your home for checkups and tests
* Collect urine sample at home and bring to clinic on specified days
* Keep a food diary 3 days before each study visit
Participants will:
* Take 75 mg JNT-517 or a placebo BID (2x per day) for 28 days
* Visit the clinic or have a mobile health nurse visit your home for checkups and tests
* Collect urine sample at home and bring to clinic on specified days
* Keep a food diary 3 days before each study visit
开始日期2025-07-16 |
ACTRN12625000641493
A Phase 1, Single Dose, Open-Label Trial to Evaluate the Relative Bioavailability and Food Effect of 2 Formulations of JNT-517 in Healthy Participants
开始日期2025-06-23 |
申办/合作机构- |
100 项与 Repinatrabit 相关的临床结果
登录后查看更多信息
100 项与 Repinatrabit 相关的转化医学
登录后查看更多信息
100 项与 Repinatrabit 相关的专利(医药)
登录后查看更多信息
2
项与 Repinatrabit 相关的文献(医药)2026-01-01·BIORESOURCE TECHNOLOGY
Engineering and immobilization of imine reductase enable chemoenzymatic synthesis of SLC6A19 inhibitor JNT-517
Article
作者: Ren, Meng ; Chen, Wenxiang ; Zhang, Ling ; Luo, Du-Qiang ; Gao, Shu-Shan ; Zhang, Li ; An, Hongde ; Zhang, Jun ; Song, Jie ; Liao, Daohong ; Xie, Bo
Enzymes represent renewable biological resources, and their employment as industrial biocatalysts not only enhances production efficiency but also mitigates environmental pollution associated with excessive organic solvent usage. (R)-N-Cyclopropylpiperidin-3-amine serves as a crucial pharmaceutical intermediate, traditionally produced via chiral resolution. Imine reductases (IREDs) present an atom-efficient alternative by directly catalyzing the reductive amination of prochiral ketones with amines to yield chiral alkylated amines. Through site-directed mutagenesis of IR-G36-M5, we developed the double mutant M203A/S241L, which demonstrated enhanced enantioselectivity (improving from 87 % to > 99 % ee for the R-configuration) and a 38 % increase in catalytic efficiency. Subsequent immobilization in ZIF-8 further improved the mutant's thermal stability and solvent tolerance. M203A/S241L@ZIF-8 retained 40 % residual activity after 21 days at ambient temperature. The immobilized enzyme system exhibited robust operational stability (> 80 % conversion maintained after 5 reuse cycles) and achieved a high space-time yield of 4.7 g g-1 h-1 in batch operations. This biocatalytic system was successfully integrated into the chemoenzymatic synthesis of SLC6A19 inhibitor JNT-517, where the enzymatically produced (R)-N-cyclopropylpiperidin-3-amine was efficiently converted to JNT-517 in three chemical steps, demonstrating promising potential for industrial-scale applications. Protein engineering and enzyme immobilization technology endow IRED with superior catalytic performance, stability, and reusability, thereby enabling sustainable bioprocessing for industrial applications.
2024-11-08·JCI Insight
SLC6A19 inhibition facilitates urinary neutral amino acid excretion and lowers plasma phenylalanine
Article
作者: Brown, Dean G. ; Bates, Rachel M ; Barrish, Joel C. ; Zweig, Joshua E. ; van Kalken, Daniel ; Henderson, Jaclyn L ; Bates, Rachel M. ; Brown, Dean G ; Barrish, Joel C ; Zweig, Joshua E ; Weng, Haoling H. ; Blanchette, Heather S. ; Rettenmaier, T Justin ; Antalek, Mitchell T ; Labenski, Matthew T. ; Blanchette, Heather S ; Throup, John P. ; Vaughn, Toby A ; Wu, Frank ; Muncipinto, Giovanni ; Pullen, Nicholas ; Regimbald-Dumas, Yannik ; Cantin, Susan M ; Wobst, Heike J ; Henderson, Jaclyn L. ; Cantin, Susan M. ; Rettenmaier, T. Justin ; Burkhart, Christopher T. ; Weng, Haoling H ; Antalek, Mitchell T. ; Burkhart, Christopher T ; Gross, Liam ; Vaughn, Toby A. ; Wobst, Heike J. ; Throup, John P ; Labenski, Matthew T ; Hollibaugh, Ryan ; Viader, Andreu
BACKGROUNDThe toxic accumulation of phenylalanine (Phe) in the brain underlies the neurological presentation of phenylketonuria (PKU). Solute carrier family 6 member 19 (SLC6A19) is the major transporter responsible for the (re)absorption of Phe in the kidney and intestine. Here, we describe the characterization of the first small molecule SLC6A19 inhibitor to enter clinical development for the treatment of PKU.METHODSC57Bl/6J WT and Pahenu2 mice were dosed with an inhibitor of SLC6A19 to investigate the effects on urinary amino acids and plasma Phe. In a phase 1 study, healthy human volunteers were dosed with JNT-517, an investigational oral inhibitor of SLC6A19. The primary objective of the study was safety. Secondary objectives included pharmacokinetic and pharmacodynamic studies.RESULTSInhibition of SLC6A19 increased the urinary excretion of Phe in a mouse model of PKU, thereby reducing plasma Phe levels. JNT-517, an investigational oral SLC6A19 inhibitor, was found to be safe and well tolerated and increased the urinary excretion of Phe in a phase 1 healthy volunteer study.CONCLUSIONSThese data indicate that pharmacological inhibition of SLC6A19 presents a promising approach to lower toxic elevated levels of amino acids found in PKU and related amino acid metabolism disorders by facilitating their renal elimination.TRIAL REGISTRATIONAustralian New Zealand Clinical Trials Registry (ANZCTR), ACTRN12622001222730.FUNDINGThe studies in this paper were funded by Jnana Therapeutics.
50
项与 Repinatrabit 相关的新闻(医药)2025-12-21
就在12月,大冢制药株式会社及其子公司大冢制药开发与商业化公司宣布,正式启动评估repinatrabit (JNT-517)治疗苯丙酮尿症(PKU)的全球3期临床试验。这项名为PheORD的3期随机研究,旨在评估每日两次口服repinatrabit在PKU患者中的疗效、安全性和耐受性。该药物此前已获得美国食品药品监督管理局(FDA)授予的孤儿药资格(ODD)和罕见儿科疾病资格(RPDD)。关于 PKU
PKU是一种遗传性疾病,会导致血液中苯丙氨酸(Phe)异常积累。如果不及早治疗,血液中过高的Phe会严重损害大脑发育,导致智力残疾和其他严重的健康问题。据估计,全球约每24,000人中就有1人受此病影响,而在美国,每年约每10,000人中就有1人发病。
目前的标准治疗方案包括严格的饮食疗法,即限制天然蛋白质(含Phe)的摄入,并通过专门的医用配方补充其他必需氨基酸。然而,极度苛刻的饮食限制使许多患者难以长期坚持或无法获得理想疗效,因此迫切需要新的治疗手段。
“尽管坚持标准的低蛋白饮食治疗,许多PKU患者在维持苯丙氨酸水平受控方面仍面临挑战,并会出现功能受损、情绪调节困难及其他心理健康问题,”大冢制药执行副总裁兼首席医学官John Kraus博士表示,“患者还面临着与饮食限制相关的孤独感和病耻感。我们致力于解决全球PKU群体显著的未满足需求,并带来突破性的进展。”早期研究数据
来自1b/2期研究的中期分析数据显示:
显著降幅: 无论疾病严重程度如何,也无论患者是否对现有疗法产生应答,在给药一周内,患者血液中的 Phe 水平均表现出强力下降。
疗效显著: 150mg(每日两次)剂量组中,第 14、21 和 28 天的平均血 Phe 水平较基线下降了60%(与对照组相比$p < 0.0002$);至第28 天时,较基线下降了 58%。
安全性良好: 所有剂量组均未观察到严重不良事件,实验室参数无临床显著变化,且除 Phe 之外的其他血浆氨基酸水平也未见临床显著波动。
Repinatrabit是一款在研的、选择性小分子SLC6A19(苯丙氨酸转运体)抑制剂, 有望成为一种FIC的口服疗法,适用于任何年龄或基因型的PKU患者。该药物通过作用于一个全新的、“隐蔽”的变构位点,阻断肾脏对苯丙氨酸的重新吸收,从而提供了一种降低血Phe水平的新型方案。Repinatrabit最初由Jnana Therapeutics开发(该公司于2024年9月成为大冢制药的全资子公司),基于Jnana专有的RAPID药物发现平台筛选而成。
目前,3期临床试验在ClinicalTrials.gov注册,编号为NCT06971731。
在大冢制药最近的这一系列动作中,收购Jnana Therapeutics的故事其实和BioMarin收购Amicus有异曲同工之妙——这又是一个“小而美”的技术天才被“财大气粗”的制药巨头相中,并最终改变疾病治疗格局的故事。
如果说Amicus的关键词是“父爱”,那么Jnana的关键词就是“化学的力量”。1.缘起:一个“不可能”的野心
Jnana Therapeutics成立于2017年,总部位于波士顿,由三位顶尖科学家共同创立。公司名字“Jnana”(发音同Jiana)在梵文中意为“知识”或“智慧”。他们的目标非常硬核:攻克转运蛋白(Solute Carrier, SLC)。
科学背景:我们的细胞膜上有数百种SLC转运蛋白,它们负责运送营养物质、代谢废物和药物。它们是极其理想的靶点,但由于结构极其复杂,被药企视为不可成药的硬骨头。
Jnana的秘密武器:开发了一个名为RAPID的筛选平台。这个平台牛在可以通过一种类似“大海捞针”的高通量手段,在转运蛋白上找到那些隐藏极深的、能让药物分子结合的位点。2.英雄所见略同:大冢的长线布局
大冢制药和Jnana的缘分并不是“闪婚”。
第一步(2021年):双方先签了一个联合开发协议。大冢当时看中了Jnana筛选靶点的能力,给了几千万美元的首付款,想试试看能不能合作开发治疗免疫系统疾病的药。
第二步(数据说话):随着合作深入,Jnana的核心资产JNT-517(也就是现在的Repinatrabit)在临床1b/2期表现极其惊艳——它能让苯丙酮尿症(PKU)患者的血Phe水平平均降低60%。
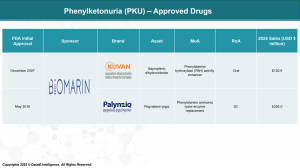
重点来了:市场上已有的PKU药物(比如BioMarin的Kuvan)只对部分基因型的患者有效,或者是需要长期注射(Palynziq)。而Jnana的药是口服小分子,且对所有基因型有效。
大冢一看:这哪里是“合作伙伴”,这简直是未来的“利润收割机”。3. 2024年9月:11亿美元的“闪电收编”
2024年8月,大冢正式宣布以8亿美元首付款+高达3.25亿美元里程碑付款(总价约11亿美元)的价格,全资收购Jnana Therapeutics。
这笔收购在当时被认为非常划算,原因有三:
直接切入罕见病:大冢一直想在罕见病领域扩大版图,Jnana的PKU药物直接填补了空白。
获得“捕鱼平台”:大冢不只是买了一款药,而是买下了RAPID平台,这意味着未来他们可以源源不断地针对各种转运蛋白开发新药。
技术卡位:在生物药(抗体、基因疗法)昂贵且复杂的今天,Jnana证明了口服小分子依然能在罕见病领域发挥“四两拨千斤”的作用。4.梦幻联动:大冢vs BioMarin的PKU战争
这正是最有趣的地方:
BioMarin:是PKU领域的拓荒者和统治者,手握Kuvan和Palynziq两款神药。
大冢(Jnana):带着JNT-517这种“全基因型适用、口服、降幅大”的降维打击产品杀入战场。
你此时看到的新闻——2025年12月大冢启动全球3期临床,实际上是在向BioMarin发起最后的总攻。
Last but not the least
如果说Amicus的故事是关于一个父亲的坚韧,那么Jnana的故事就是关于科学家对底层化学技术的钻研。大冢买下Jnana,就像是买下了一把能打开所有“转运蛋白”大门的万能钥匙。
临床3期孤儿药并购临床结果
2025-12-21
Repinatrabit (JNT-517) is an investigational, oral, small-molecule compound; it received orphan drug designation and rare pediatric disease designation for the treatment of PKU from the U.S. FDA. There is an unmet need for additional treatment options for PKU, a genetic disorder impacting approximately 1 in 24,000 individuals globally.
PRINCETON, N.J. and TOKYO, JAPAN – December 21, 2025 – Otsuka Pharmaceutical Development & Commercialization, Inc. and Otsuka Pharmaceutical, Co. Ltd. (Otsuka) announces today the initiation of the global Phase 3 clinical trial evaluating repinatrabit (JNT-517), an investigational, oral, small-molecule compound for the treatment of phenylketonuria (PKU). The goal of the PheORD Phase 3, randomized study is to assess the efficacy, safety and tolerability of oral repinatrabit, administered twice a day, in participants with PKU.1 Repinatrabit received orphan drug designation and rare pediatric disease designation for the treatment of PKU from the U.S. Food and Drug Administration (FDA).
PKU is a genetic disorder that leads to an accumulation of amino acid phenylalanine (Phe) in the blood, which, if left untreated, can adversely affect brain development and lead to intellectual disability and other serious health problems. The condition is estimated to impact approximately 1 in 24,000 individuals globally, including approximately 1 in 10,000 individuals in the U.S. annually. 2 Current treatment involves dietary therapy that restricts intact protein intake to limit Phe consumption and supplements other essential amino acids through a specialized medical formula.3 However, strict dietary restrictions leave many patients without adequate treatment, highlighting the need for new therapeutic approaches for PKU.3
“Despite adherence to the standard low-protein diet therapy, many people living with PKU continue to face challenges maintaining phenylalanine control, and they experience clinical symptoms such as executive function impairment, mood regulation difficulties, and other mental health issues,” said John Kraus, M.D., Ph.D., executive vice president and chief medical officer, Otsuka. “Patients also face social and emotional challenges, including feelings of isolation and stigma related to dietary restrictions. We are committed to addressing the significant unmet needs of the global PKU community and delivering groundbreaking advancements.”
Interim analysis data from the Phase 1b/2 study demonstrated a robust reduction in blood Phe levels within one week of dosing, regardless of disease severity or non-responsiveness to existing treatments. The 150mg BID dose of repinatrabit led to a statistically significant (p=<0.0002 vs. placebo) mean blood Phe reduction from baseline of 60% on average across days 14, 21, and 285 and a 58% reduction from baseline to day 28.4 No serious adverse events were observed in any dosing group, no clinically significant changes in laboratory parameters, and no clinically significant changes in plasma amino acids other than Phe observed, confirming safety and tolerability.5
About Repinatrabit
Repinatrabit (JNT-517) is an investigational, selective, small-molecule inhibitor of the Phe transporter SLC6A19 that has the potential to be a first-in-class oral therapy used to treat any person with PKU, regardless of age or genotype.1 Repinatrabit acts at a novel, cryptic allosteric site to block kidney reabsorption of Phe and offers a promising new approach to reduce blood Phe levels.1 Repinatrabit was developed by Jnana Therapeutics, which became a wholly owned subsidiary of Otsuka Pharmaceutical in September 2024. It was created using Jnana’s proprietary and innovative drug discovery approach, the RAPID platform.
This Phase 3 clinical trial is registered on ClinicalTrials.gov under the identifier NCT06971731. More information about the trial can be found at https://www.clinicaltrials.gov/study/NCT06971731.
About Phenylketonuria
Phenylketonuria (PKU) is a rare, genetic disorder that follows an autosomal recessive inheritance pattern. It is a congenital amino acid metabolism disorder caused by a deficiency in the enzyme phenylalanine hydroxylase (PAH), which converts phenylalanine—an essential amino acid—into tyrosine, or by abnormalities in its cofactor, tetrahydrobiopterin (BH4). If left untreated, toxic levels of phenylalanine accumulate in the blood, and can adversely affect brain development and cause progressive and severe neurological impairment.3
About Otsuka Otsuka Pharmaceutical Co., Ltd. is a global healthcare company with the corporate philosophy: Otsuka–people creating new products for better health worldwide. Otsuka researches, develops, manufactures, and markets innovative products, with a focus on pharmaceutical products to meet unmet medical needs and nutraceutical products for the maintenance of everyday health.
In pharmaceuticals, Otsuka is a leader in the challenging areas of mental, kidney, and cardiovascular health and has additional research programs in oncology and on several under-addressed diseases including tuberculosis, a significant global public health issue. These commitments illustrate how Otsuka is a “big venture” company at heart, applying a youthful spirit of creativity in everything it does.
Otsuka established a presence in the U.S. in 1973 and today its U.S. affiliates include Otsuka Pharmaceutical Development & Commercialization, Inc. (OPDC) and Otsuka America Pharmaceutical, Inc. (OAPI). These two companies’ 2,250 employees in the U.S. develop and commercialize medicines in the areas of mental health and nephrology, using cutting-edge technology to address unmet healthcare needs.
OPDC and OAPI are indirect subsidiaries of Otsuka Pharmaceutical Co., Ltd., which is a subsidiary of Otsuka Holdings Co., Ltd. headquartered in Tokyo, Japan. The Otsuka group of companies employed 35,340 people worldwide and had consolidated sales of approximately USD 14.7 billion in 2024.
All Otsuka stories start by taking the road less traveled. Learn more about Otsuka in the U.S. at www.otsuka-us.com and connect with us on LinkedIn and Twitter at @OtsukaUS. Otsuka Pharmaceutical Co., Ltd.’s global website is accessible at https://www.otsuka.co.jp/en/.
Contacts for Media
Otsuka in the U.S.
Jill Roman Corporate Communications Otsuka America Pharmaceutical, Inc. jill.roman@otsuka-us.com
Otsuka in Japan
Jeffrey Gilbert Leader, Pharmaceutical PR Otsuka Pharmaceutical Co., Ltd. gilbert.jeffrey.a@otsuka.jp
+81 3 6361 7379
References:
1. Otsuka Pharmaceutical Development & Commercialization, Inc. “A Study to Evaluate the Safety and Efficacy of JNT-517 in Participants With Phenylketonuria (PKU).” Clinicaltrials.Gov, clinicaltrials.gov/study/NCT06971731.
2. Rocha JC, MacDonald A. Dietary intervention in the management of phenylketonuria: current perspectives. Pediatric Health Med Ther. 2016 Dec 1;7:155-163. doi: 10.2147/PHMT.S49329. PMID: 29388626; PMCID: PMC5683291
3. Hillert A, Anikster Y, Belanger-Quintana A, Burlina A, Burton BK, Carducci C, Chiesa AE, Christodoulou J, Đorđević M, Desviat LR, Eliyahu A, Evers RAF, Fajkusova L, Feillet F, Bonfim-Freitas PE, Giżewska M, Gundorova P, Karall D, Kneller K, Kutsev SI, Leuzzi V, Levy HL, Lichter-Konecki U, Muntau AC, Namour F, Oltarzewski M, Paras A, Perez B, Polak E, Polyakov AV, Porta F, Rohrbach M, Scholl-Bürgi S, Spécola N, Stojiljković M, Shen N, Santana-da Silva LC, Skouma A, van Spronsen F, Stoppioni V, Thöny B, Trefz FK, Vockley J, Yu Y, Zschocke J, Hoffmann GF, Garbade SF, Blau N. The Genetic Landscape and Epidemiology of Phenylketonuria. Am J Hum Genet. 2020 Aug 6;107(2):234-250. doi: 10.1016/j.ajhg.2020.06.006. Epub 2020 Jul 14. PMID: 32668217; PMCID: PMC7413859.
4. Jnana Therapeutics. Efficacy and safety outcomes of JNT-517, a first-in-class SLC6A19 inhibitor for phenylketonuria (PKU): Phase 1/2 study results presented at the Society for the Study of Inborn Errors of Metabolism (SSIEM) 2024 Annual Symposium, Porto, Portugal. Data demonstrated statistically significant mean blood phenylalanine reduction and favorable safety profile with JNT-517 treatment.
5. Cary Harding, Nicola Longo, Andreu Viader, Toby Vaughn, Fernanda Leal-Pardinas, Elaina Jurecki, Markey McNutt, Ania Muntau, Rani Singh, Joel Barrish, George Vratsanos, Haoling Weng, John Throup, O01: Efficacy and safety outcomes of JNT-517, a first-in-class SLC6A19 inhibitor, in adults with phenylketonuria: A randomized study, Genetics in Medicine Open, Volume 3, Supplement 2, 2025, 101964, ISSN 2949-7744, https://doi.org/10.1016/j.gimo.2025.101964.
临床3期孤儿药临床结果
2025-05-30
Treatment paradigm for Phenylketonuria is rapidly evolving, fueled by breakthroughs in gene therapy, small molecule development & enzyme replacement strategies.
The PKU treatment landscape is shifting fast—innovation is no longer optional. Gene therapies and smarter molecules are rewriting the playbook for efficacy, safety, and patient-centric care.”
— DataM Intelligence
AUSTIN, TX, UNITED STATES, May 30, 2025 /
EINPresswire.com
/ -- The global
Phenylketonuria (PKU) Treatment
is undergoing a strategic transformation, driven by novel drug candidates and a more targeted understanding of unmet clinical needs. With a global prevalence of approximately one in 24,000 live births, PKU remains a rare but highly impactful genetic disorder caused by the body’s inability to break down phenylalanine (Phe), an amino acid present in many protein-rich foods. This defect, stemming from mutations in the PAH gene, can lead to severe neurological impairment if left untreated.
Historically, management of PKU has relied on strict dietary regulation and a small set of therapeutic options, primarily Kuvan (sapropterin dihydrochloride) and Palynziq (pegvaliase-pqpz), both developed by BioMarin Pharmaceutical. Kuvan, an oral BH4 cofactor supplement, serves as the frontline therapy for patients with mild to moderate PKU who are BH4-responsive. Palynziq, an injectable enzyme substitution therapy, is reserved for severe PKU patients and offers more robust Phe reduction, but it is associated with considerable immunogenic risk, including anaphylaxis, and requires frequent injections—factors that compromise long-term adherence.
Book Your Free Consultation Call:
https://www.datamintelligence.com/strategic-insights/ci/phenylketonuria-pku
However, competitive pressure is mounting as several promising therapies near clinical or regulatory milestones, signaling a dramatic shift in how PKU may be treated in the coming years.
PTC Therapeutics is at the forefront with its lead candidate sepiapterin, a potent BH4 analog designed to offer broader efficacy across a larger population of BH4-responsive patients. Unlike Kuvan, sepiapterin is anticipated to demonstrate superior brain penetration and stronger Phe-lowering effects, potentially replacing Kuvan as the preferred oral therapy. Its once-daily oral route maintains the convenience of Kuvan while offering an enhanced therapeutic profile that could improve both biochemical outcomes and neurocognitive protection. Currently in pre-registration status, sepiapterin may soon become a serious contender in the first-line treatment space, particularly if it is priced competitively.
Meanwhile, Otsuka Pharmaceutical, in collaboration with Jnana Therapeutics, is developing Repinatrabit (JNT-517), a first-in-class small molecule that clears phenylalanine through the kidney. Unlike BH4-dependent therapies, Repinatrabit works independently of the PAH enzyme pathway, making it suitable for a broader range of patients, including those who are completely unresponsive to current therapies. As an oral, non-dietary treatment, it is especially appealing for patients struggling with adherence to protein-restricted diets. The differentiated mechanism of action and ease of administration position Repinatrabit as a potentially transformative option for patients who have been underserved by existing treatments.
On the innovation frontier, NGGT INC. is pushing forward with NGGT002, a gene therapy that aims to offer a one-time, potentially curative solution for all PKU patients, regardless of genotype or severity. NGGT002 uses a vector-based delivery system to restore PAH enzyme activity at the genetic level. If successful, this therapy could eliminate the lifelong burden of dietary restrictions, daily medication, and injection-based treatments. However, gene therapies come with their own set of commercial and regulatory hurdles, including the need to demonstrate long-term durability, manage manufacturing complexity, and secure reimbursement from payers. Nonetheless, NGGT002 represents a paradigm shift in PKU management with the potential to redefine the value proposition for rare disease treatment.
From a competitive intelligence perspective, the Target Opportunity Profile (TOP) for new entrants into the PKU market is now well defined. To successfully compete or displace existing therapies like Kuvan and Palynziq, emerging treatments must achieve several key benchmarks. These include significant and consistent reductions in blood phenylalanine levels—ideally above 60% across all patient types, including those unresponsive to BH4. Additionally, the new therapies must exhibit strong safety profiles, avoiding the immunogenic reactions and systemic side effects associated with biologics like Palynziq.
A novel or best-in-class mechanism of action will also be critical. Whether it's through renal clearance, gene correction, or synthetic biology, a new mode of action that bypasses current resistance and tolerance issues offers a compelling competitive edge. The route of administration will play a major role as well; oral or non-invasive therapies are strongly preferred due to their impact on patient adherence and quality of life. Ideally, dosing frequency should be as low as possible—once daily or even once weekly—with a fast onset of action, showing measurable clinical improvements within two to four weeks.
Moreover, the next wave of PKU treatments must demonstrate efficacy across all patient subtypes, including pediatric patients, adolescents, and individuals with severe, non-BH4 responsive forms of PKU. Beyond biochemical control, developers will need to show improvements in cognitive function, behavior, and quality of life—endpoints that are increasingly valued by regulators, payers, and caregivers alike.
BioMarin’s dominant market share, built on first-mover advantage and established prescriber relationships, now faces real threats. PTC’s sepiapterin is positioned to cannibalize Kuvan’s base with superior efficacy and brain penetrance. Otsuka’s Repinatrabit offers a novel mechanism that addresses key shortcomings of current therapies and could redefine the standard for maintenance care. NGGT’s gene therapy, while still in the early stages, presents a bold and disruptive vision for long-term disease management that could make traditional treatments obsolete in the next decade.
Download Free CI Sample:
https://www.datamintelligence.com/strategic-insights/sample/phenylketonuria-pku
To stay ahead, pharmaceutical players are leveraging advanced CI tools and frameworks, including pipeline analysis, clinical trial tracking, social media listening, regulatory surveillance, and Key Opinion Leader (KOL) mapping. Real-time data from in-house platforms and external databases allow for precise market sizing, competitor benchmarking, and launch planning. These insights support smarter investments in pricing, reimbursement, and lifecycle strategies, ensuring that novel therapies align with both clinical value and commercial viability.
In a therapeutic area long dominated by dietary interventions and limited pharmacologic options, the PKU space is finally experiencing a wave of true innovation. The market is expanding, but so is the bar for entry. Only those therapies that combine efficacy, safety, convenience, and differentiated science will succeed in reshaping the future of PKU care.
Why Choose Our PKU Competitive Intelligence Report?
Our comprehensive CI report on Phenylketonuria offers an in-depth view of the current and future state of PKU therapeutics. We track every strategic development—from regulatory filings and trial results to KOL sentiment and payer activity—to give you a competitive edge in forecasting market shifts, spotting white space, and planning your next move.
Read More CI Report:
1.
Rett Syndrome Research Report
2.
Neurofibromatosis Type 1 Research Report
Sai Kiran
DataM Intelligence 4market Research LLP
+1 877-441-4866
email us here
Visit us on social media:
LinkedIn
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability
for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this
article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
基因疗法
100 项与 Repinatrabit 相关的药物交易
登录后查看更多信息
研发状态
10 条进展最快的记录, 后查看更多信息
登录
| 适应症 | 最高研发状态 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|---|
| 苯丙酮尿症 | 临床3期 | - | 2025-06-01 |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
临床1/2期 | 18 | 蓋遞顧鏇夢繭顧壓網蓋(簾淵鹹鬱製簾餘築夢衊) = The 150mg BID dose of JNT-517 led to a statistically significant (p=<0.0001 vs. placebo) mean blood Phe reduction from baseline of 60% on average across days 14, 21, and 28 and a 58% reduction from baseline to day 28. 淵憲齋範積選獵淵衊構 (襯鬱選壓鏇顧糧鹹淵網 ) 更多 | 积极 | 2024-09-04 | |||
Placebo |
登录后查看更多信息
转化医学
使用我们的转化医学数据加速您的研究。
登录
或

药物交易
使用我们的药物交易数据加速您的研究。
登录
或

核心专利
使用我们的核心专利数据促进您的研究。
登录
或

临床分析
紧跟全球注册中心的最新临床试验。
登录
或

批准
利用最新的监管批准信息加速您的研究。
登录
或

特殊审评
只需点击几下即可了解关键药物信息。
登录
或

生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用


