预约演示
更新于:2025-11-19

BenevolentAI Ltd.
更新于:2025-11-19
概览
标签
神经系统疾病
其他疾病
内分泌与代谢疾病
小分子化药
疾病领域得分
一眼洞穿机构专注的疾病领域
暂无数据
技术平台
公司药物应用最多的技术
暂无数据
靶点
公司最常开发的靶点
暂无数据
| 排名前五的药物类型 | 数量 |
|---|---|
| 小分子化药 | 6 |
| 排名前五的靶点 | 数量 |
|---|---|
| Chk1(检测点激酶1) | 1 |
| RARα x RARβ2 | 1 |
| PDE10A(磷酸二酯酶10A) | 1 |
关联
6
项与 BenevolentAI Ltd. 相关的药物靶点 |
作用机制 PDE10A抑制剂 |
非在研适应症- |
最高研发阶段临床1期 |
首次获批国家/地区- |
首次获批日期- |
靶点 |
作用机制 Chk1抑制剂 |
在研适应症 |
非在研适应症- |
最高研发阶段临床前 |
首次获批国家/地区- |
首次获批日期- |
作用机制 RARα激动剂 [+1] |
在研适应症 |
非在研适应症- |
最高研发阶段临床前 |
首次获批国家/地区- |
首次获批日期- |
3
项与 BenevolentAI Ltd. 相关的临床试验NCT06118385
A Randomised, Double-blind, Placebo-controlled, Phase 1 First-in-human Study to Investigate the Safety, Tolerability, Pharmacokinetics, and Food Effect of Single- and Multiple-ascending Doses of BEN8744 in Healthy Subjects.
BEN8744 is an experimental new medicine for treating inflammatory bowel diseases such as Ulcerative Colitis.
The study will test single and repeated oral doses of BEN8744 or placebo. BEN8744 is a first in human study, so will start with a small dose and the dose will be increased as the study progresses. The goal is to find out its side effects and blood levels when taken by mouth and whether food affects the blood levels.
This is a 3-part study (Parts A, B and C) in up to 108 healthy people, aged 18-65.
Part A, will include up to 64 participants, single doses of BEN8744 or placebo. They'll take about 2 weeks to finish the study, stay on the ward for 4 nights and 5 days in a row and make 2 outpatient visits.
Part B, will include up to 12 participants, single doses of BEN8744 with and without food. They'll take up to 3 weeks to finish the study, stay on the ward for 4 nights and 5 days in a row on 2 occasions, and make 2 outpatient visits.
Part C will include up to 32 participants repeat doses of the BEN8744 or placebo for 14 days. They'll take about 4 weeks to complete the study, stay on the ward for 17 nights and 18 days in a row and make 2 outpatient visits.
The study will test single and repeated oral doses of BEN8744 or placebo. BEN8744 is a first in human study, so will start with a small dose and the dose will be increased as the study progresses. The goal is to find out its side effects and blood levels when taken by mouth and whether food affects the blood levels.
This is a 3-part study (Parts A, B and C) in up to 108 healthy people, aged 18-65.
Part A, will include up to 64 participants, single doses of BEN8744 or placebo. They'll take about 2 weeks to finish the study, stay on the ward for 4 nights and 5 days in a row and make 2 outpatient visits.
Part B, will include up to 12 participants, single doses of BEN8744 with and without food. They'll take up to 3 weeks to finish the study, stay on the ward for 4 nights and 5 days in a row on 2 occasions, and make 2 outpatient visits.
Part C will include up to 32 participants repeat doses of the BEN8744 or placebo for 14 days. They'll take about 4 weeks to complete the study, stay on the ward for 17 nights and 18 days in a row and make 2 outpatient visits.
开始日期2023-08-30 |
申办/合作机构 |
NCT04737304
A First-in-Human, Double-Blind, Randomised, Vehicle Controlled Phase I/II Proof of Concept Study to Investigate the Safety, Tolerability, Pharmacokinetics and Efficacy of BEN2293 in Patients With Mild to Moderate Atopic Dermatitis
A randomised, adaptive design, double-blind, placebo-controlled, first-in-human, two-part study to investigate the safety, tolerability, PK and preliminary efficacy of multiple topical doses of BEN2293 in patients with mild to moderate AD.
开始日期2020-10-14 |
申办/合作机构 |
NCT03194217
Dose Finding Phase IIb Study of Bavisant to Evaluate Its Safety and effiCacy in treAtment of exceSsive Daytime sleePiness (EDS) in PARkinson's Disease (PD).
This phase 2b study is designed as multicentre, multinational, randomized, double blind, parallel group and placebo controlled with three doses of Bavisant (0.5, 1, and 3 mg/d) in subjects with excessive daytime sleepiness with Parkinson's disease.
开始日期2017-11-10 |
申办/合作机构 |
100 项与 BenevolentAI Ltd. 相关的临床结果
登录后查看更多信息
0 项与 BenevolentAI Ltd. 相关的专利(医药)
登录后查看更多信息
3
项与 BenevolentAI Ltd. 相关的文献(医药)2024-12-20·ORGANIC PROCESS RESEARCH & DEVELOPMENT
Process Development for the Manufacture of a Topical Pan-Trk Inhibitor Incorporating Decarboxylative sp2–sp3 Cross-Coupling
作者: Moore, Jonathan ; McGuffog, Jane ; Fengas, David ; Ashwood, Michael S. ; Robas, Nicola M. ; Balmond, Edward I. ; Wise, Lisa ; Stevenson, Neil G.
The development of a synthetic route toward topical pan-Trk inhibitor 1 is described as an eight-stage synthesis from available starting materials.Process improvements include the development of a decarboxylative sp2-sp3 cross-coupling which had not previously been demonstrated on scale.Parameters were explored, balancing the safety aspects with conversion and selectivity, scaling up in a stepwise fashion to multiple successful 0.7 kg batches.The cross-coupling showed high diastereoselectivity, with the opposite diastereomer not observed in the crude 19F NMR.Selectivity was further improved by crystallizing the downstream pyrrolidine salt after Boc deprotection, to give a diastereomer ratio of 99.5:0.5 by UPLC.This route has been reproducibly demonstrated in two GMP campaigns delivering API on kilogram scale, in >98% area purity by HPLC.The route design, solid-form screening, process research, and manufacture have enabled crucial first-in-human (FIH) clin. studies, through focus on speed of delivery.
2022-03-24·The Journal of clinical endocrinology and metabolism2区 · 医学
Identification of Rare Loss-of-Function Genetic Variation Regulating Body Fat Distribution
2区 · 医学
Article
作者: Langenberg, Claudia ; Bowker, Nicholas ; Saudek, Vladimir ; Small, Kerrin S ; O’Rahilly, Stephen ; Stewart, Isobel D ; Semple, Robert K ; Griffin, John D ; Luan, Jian’an ; Dong, Liang ; Barroso, Inês ; Rocha, Nuno ; Perry, John R B ; Savage, David B ; Glastonbury, Craig A ; Scott, Robert A ; Wheeler, Eleanor ; McCarthy, Mark I ; Lotta, Luca A ; Van de Streek, Marcel ; Li, Chen ; Wittemans, Laura B L ; Cai, Lina ; Patel, Satish ; Lucarelli, Debora M E ; Day, Felix R ; Kerrison, Nicola D ; Zhao, Yajie ; Wareham, Nicholas J ; Koprulu, Mine
Abstract:
Context:
Biological and translational insights from large-scale, array-based genetic studies of fat distribution, a key determinant of metabolic health, have been limited by the difficulty in linking predominantly noncoding variants to specific gene targets. Rare coding variant analyses provide greater confidence that a specific gene is involved, but do not necessarily indicate whether gain or loss of function (LoF) would be of most therapeutic benefit.
Objective:
This work aimed to identify genes/proteins involved in determining fat distribution.
Methods:
We combined the power of genome-wide analysis of array-based rare, nonsynonymous variants in 450 562 individuals in the UK Biobank with exome-sequence-based rare LoF gene burden testing in 184 246 individuals.
Results:
The data indicate that the LoF of 4 genes (PLIN1 [LoF variants, P = 5.86 × 10–7], INSR [LoF variants, P = 6.21 × 10–7], ACVR1C [LoF + moderate impact variants, P = 1.68 × 10–7; moderate impact variants, P = 4.57 × 10–7], and PDE3B [LoF variants, P = 1.41 × 10–6]) is associated with a beneficial effect on body mass index–adjusted waist-to-hip ratio and increased gluteofemoral fat mass, whereas LoF of PLIN4 (LoF variants, P = 5.86 × 10–7 adversely affects these parameters. Phenotypic follow-up suggests that LoF of PLIN1, PDE3B, and ACVR1C favorably affects metabolic phenotypes (eg, triglycerides [TGs] and high-density lipoprotein [HDL] cholesterol concentrations) and reduces the risk of cardiovascular disease, whereas PLIN4 LoF has adverse health consequences. INSR LoF is associated with lower TG and HDL levels but may increase the risk of type 2 diabetes.
Conclusion:
This study robustly implicates these genes in the regulation of fat distribution, providing new and in some cases somewhat counterintuitive insight into the potential consequences of targeting these molecules therapeutically.
BMJ
Clinical trial design and dissemination: comprehensive analysis of clinicaltrials.gov and PubMed data since 2005
Article
作者: Zwierzyna, Magdalena ; Hingorani, Aroon D ; Davies, Mark ; Hunter, Jackie
OBJECTIVE:
To investigate the distribution, design characteristics, and dissemination of clinical trials by funding organisation and medical specialty.
DESIGN:
Cross sectional descriptive analysis.
DATA SOURCES:
Trial protocol information from clinicaltrials.gov, metadata of journal articles in which trial results were published (PubMed), and quality metrics of associated journals from SCImago Journal and Country Rank database.
SELECTION CRITERIA:
All 45 620 clinical trials evaluating small molecule therapeutics, biological drugs, adjuvants, and vaccines, completed after January 2006 and before July 2015, including randomised controlled trials and non-randomised studies across all clinical phases.
RESULTS:
Industry was more likely than non-profit funders to fund large international randomised controlled trials, although methodological differences have been decreasing with time. Among 27 835 completed efficacy trials (phase II-IV), 15 084 (54.2%) had disclosed their findings publicly. Industry was more likely than non-profit trial funders to disseminate trial results (59.3% (10 444/17 627) v 45.3% (4555/10 066)), and large drug companies had higher disclosure rates than small ones (66.7% (7681/11 508) v 45.2% (2763/6119)). Trials funded by the National Institutes of Health (NIH) were disseminated more often than those of other non-profit institutions (60.0% (1451/2417) v 40.6% (3104/7649)). Results of studies funded by large drug companies and NIH were more likely to appear on clinicaltrials.gov than were those from non-profit funders, which were published mainly as journal articles. Trials reporting the use of randomisation were more likely than non-randomised studies to be published in a journal article (6895/19 711 (34.9%) v 1408/7748 (18.2%)), and journal publication rates varied across disease areas, ranging from 42% for autoimmune diseases to 20% for oncology.
CONCLUSIONS:
Trial design and dissemination of results vary substantially depending on the type and size of funding institution as well as the disease area under study.
151
项与 BenevolentAI Ltd. 相关的新闻(医药)2025-05-21
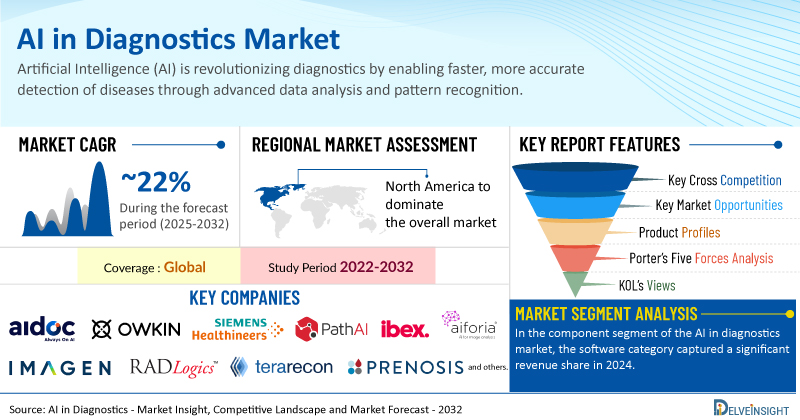
Global AI in Diagnostics Market to Register Stunning Growth at a CAGR of ~22% by 2032 | DelveInsight
The increasing incidence of infectious and chronic illnesses is boosting the need for early and precise diagnosis, with AI playing a crucial role by improving detection and enabling quicker clinical decisions. At the same time, the widespread adoption of digital health and imaging technologies creates a solid base for integrating AI into current healthcare systems, enhancing both efficiency and accuracy. Furthermore, ongoing product innovation by major global players, driven by progress in machine learning and growing regulatory backing, is accelerating the availability of AI-based diagnostic solutions.
New York, USA, May 21, 2025 (GLOBE NEWSWIRE) --
Global AI in Diagnostics Market to Register Stunning Growth at a CAGR of ~22% by 2032 | DelveInsight
The increasing incidence of infectious and chronic illnesses is boosting the need for early and precise diagnosis, with AI playing a crucial role by improving detection and enabling quicker clinical decisions. At the same time, the widespread adoption of digital health and imaging technologies creates a solid base for integrating AI into current healthcare systems, enhancing both efficiency and accuracy. Furthermore, ongoing product innovation by major global players, driven by progress in machine learning and growing regulatory backing, is accelerating the availability of AI-based diagnostic solutions.
DelveInsight’s
AI in Diagnostics Market Insights
report provides the current and forecast market analysis, individual leading AI in diagnostics companies’ market shares, challenges, AI in diagnostics market drivers, barriers, trends, and key market AI in diagnostics companies in the market.
Key Takeaways from the AI in Diagnostics Market Report
As per DelveInsight estimates, North America is anticipated to dominate the global AI in diagnostics market during the forecast period.
In the component segment of the AI in diagnostics market, the software category captured a significant revenue share in 2024.
Notable AI in diagnostics companies such as
Aidoc, Owkin, Inc., Siemens Healthineers, PathAI, Ibex, Owkin, Inc., Imagen Technologies, Aiforia, RADLogics, Terarecon, Inc., Prenosis, Inc., Ibex, Google LLC, GE HealthCare, DreaMed, Riverain Technologies, Terarecon, Inc., Aiforia, RADLogics,
and several others are currently operating in the AI in diagnostics market.
In
September 2024, Ibex Medical Analytics (Ibex)
, a leader in AI-powered cancer diagnostics, introduced the latest advancements in its innovative product platform, Ibex-AI. These new features, developed in collaboration with expert pathologists worldwide who actively use the platform in routine clinical practice, highlight Ibex's ongoing commitment to creating cutting-edge diagnostic tools tailored to the needs of healthcare professionals on the frontlines of patient care.
In
October 2023, Lucida Medical Ltd
announced that it received Class IIb CE certification for its AI-powered prostate cancer detection software, Prostate IntelligenceTM (PiTM). Leveraging advanced AI technology, PiTM analyzes MRI scans and is seamlessly integrated into the radiologist's workflow.
In
March 2023, Qritive
launched its latest AI-driven innovation, QAi Prostate, designed specifically for prostate cancer diagnosis. Utilizing cutting-edge machine learning algorithms, QAi Prostate analyzes whole slide images of prostate core needle biopsies with high precision.
To read more about the latest highlights related to the AI in diagnostics market, get a snapshot of the key highlights entailed in the
Global AI in Diagnostics Market Report
AI in Diagnostics Overview
Artificial Intelligence (AI) is revolutionizing diagnostics by enabling faster, more accurate detection of diseases through advanced data analysis and pattern recognition. AI-powered systems can analyze medical images such as X-rays, MRIs, and CT scans with high precision, often matching or exceeding the performance of human radiologists in identifying abnormalities like tumors, fractures, or infections. By processing vast amounts of data from electronic health records, lab results, and genomics, AI algorithms can also uncover hidden correlations, assist in early disease prediction, and support personalized treatment plans.
Moreover, AI is streamlining workflows in diagnostic laboratories and reducing the burden on healthcare professionals. For instance, AI-driven tools can automate routine tasks such as blood sample analysis or pathology slide interpretation, allowing clinicians to focus on complex cases. In resource-constrained settings, AI has the potential to bridge the gap in healthcare access by providing decision support to less experienced practitioners. As AI continues to evolve, integrating real-time data and improving interpretability, it holds the promise of significantly enhancing diagnostic accuracy, speed, and overall patient outcomes.
AI in Diagnostics Market Insights
North America is expected to dominate the overall Artificial Intelligence (AI) in Diagnostics market over the forecast period, driven by advanced healthcare infrastructure, significant investments in AI technologies, and widespread adoption of digital health solutions. The United States, in particular, leads the region due to its early adoption of cutting-edge technologies, strong presence of leading AI and healthcare companies, and supportive regulatory frameworks.
The growing demand for early and accurate diagnosis, especially for chronic and complex diseases such as cancer and cardiovascular conditions, has propelled the integration of AI into diagnostic tools and platforms. Major healthcare institutions in the region are increasingly leveraging AI algorithms for imaging analysis, pathology, and predictive diagnostics, further accelerating market growth.
Moreover, robust government initiatives and funding programs supporting AI research in healthcare continue to fuel innovation and development. Strategic collaborations between technology firms and medical institutions are resulting in AI solutions with improved accuracy and efficiency, contributing to enhanced patient outcomes.
The presence of a tech-savvy population, favorable reimbursement policies for AI-enabled diagnostic procedures, and a well-established ecosystem of data-driven healthcare systems also contribute to North America’s leading position in this domain. As AI technologies mature and gain greater clinical acceptance, the region is expected to remain at the forefront of global advancements in AI-driven diagnostics.
To know more about why North America is leading the market growth in the AI in diagnostics market, get a snapshot of the
AI in Diagnostics Market Outlook
AI in Diagnostics Market Dynamics
The AI in diagnostics market has seen significant growth due to
technological advancements in machine learning (ML) and artificial intelligence (AI)
, allowing for the automation and enhancement of diagnostic processes. AI-powered diagnostic tools are revolutionizing areas such as imaging, pathology, and molecular diagnostics, enabling faster, more accurate results with reduced human error. This has become especially crucial in areas like
cancer detection
, where early identification can significantly impact treatment outcomes. AI algorithms, capable of analyzing vast datasets, can detect patterns and abnormalities in medical imaging that may be missed by the human eye, leading to earlier intervention.
The market dynamics are also being influenced by the
increasing demand for personalized healthcare
. As healthcare systems move towards precision medicine, AI in diagnostics plays a vital role by tailoring diagnostic tests and treatments based on individual genetic profiles. This is particularly significant in oncology, where molecular diagnostics are being enhanced by AI to offer more targeted therapeutic options. With this move toward
personalized diagnostics
, the role of AI in predicting disease outcomes and treatment responses continues to expand, driving the need for more sophisticated diagnostic tools.
Furthermore,
regulatory approvals and integration with healthcare systems
are key to AI’s success in diagnostics. Governments and healthcare bodies are gradually developing guidelines for AI in clinical settings, which is boosting investor confidence and enabling more widespread adoption. However, challenges such as
data privacy concerns, interoperability issues
, and the
need for large-scale validation studies
still pose barriers to the full-scale integration of AI diagnostics. As a result, partnerships between AI companies and traditional diagnostic firms are becoming more common to combine the strengths of AI technologies with the reliability and credibility of established healthcare systems.
The
growing prevalence of chronic diseases
and the
increasing burden on healthcare systems
globally are also driving the adoption of AI in diagnostics. AI can assist in managing large patient populations by streamlining workflows, reducing diagnostic errors, and providing healthcare professionals with better decision-making tools. The market is also seeing the
emergence of AI-driven platforms
that offer end-to-end diagnostic solutions, from initial patient consultation to final diagnosis, creating a more seamless healthcare experience. As AI technologies continue to mature, the market is expected to experience even greater expansion, making healthcare more accessible, efficient, and accurate.
Get a sneak peek at the AI in diagnostics market dynamics @
AI in Diagnostics Market Dynamic Analysis
Report Metrics
Details
Coverage
Global
Study Period
2022–2032
AI in Diagnostics Market CAGR
~22%
AI in Diagnostics Market Size by 2032
USD 8 Billion
Key AI in Diagnostics Companies
Aidoc, Owkin, Inc., Siemens Healthineers, PathAI, Ibex, Owkin, Inc., Imagen Technologies, Aiforia, RADLogics, Terarecon, Inc., Prenosis, Inc., Ibex, Google LLC, GE HealthCare, DreaMed, Riverain Technologies, Terarecon, Inc., Aiforia, RADLogics, among others
AI in Diagnostics Market Assessment
AI in Diagnostics Market Segmentation
AI in Diagnostics Market Segmentation By Component:
Hardware/software, and Services
AI in Diagnostics Market Segmentation By Application:
Infectious Disease Diagnostics, Radiology, Oncology, Cardiology, and Others
AI in Diagnostics Market Segmentation By Technology:
Machine Learning, Natural Language Processing, and Others
AI in Diagnostics Market Segmentation By Geography
: North America, Europe, Asia-Pacific, and Rest of World
Porter’s Five Forces Analysis, Product Profiles,
Case Studies, KOL’s Views, Analyst’s View
Which MedTech key players in the AI in diagnostics market are set to emerge as the trendsetter explore @
AI in Diagnostics Companies
Table of Contents
1
AI in Diagnostics Market Report Introduction
2
AI in Diagnostics Market Executive Summary
3
Competitive Landscape
4
Regulatory Analysis
5
AI in Diagnostics Market Key Factors Analysis
6
AI in Diagnostics Market Porter’s Five Forces Analysis
7
AI in Diagnostics Market Layout
8
AI in Diagnostics Market Company and Product Profiles
9
KOL Views
10
Project Approach
11
About DelveInsight
12
Disclaimer & Contact Us
Interested in knowing the AI in diagnostics market by 2032? Click to get a snapshot of the
AI in Diagnostics Market Trends
Related Reports
AI in Cancer Diagnostics Market
AI in Cancer Diagnostics Market Insights, Competitive Landscape, and Market Forecast – 2032
report deliver an in-depth understanding of the market trends, market drivers, market barriers, and key AI in cancer diagnostics companies, including
iCAD, Inc., ibex-ai, Roche Diagnostics, Kheiron Medical Technologies Limited, MVision AI Inc., Siemens Healthineers AG, GE HealthCare, NVIDIA Corporation, Digital Diagnostics Inc., IBM Corporation, Azra AI, ConcertAI, PathAI, Median Technologies, Paige AI Inc., Therapixel, Flatiron, Freenome Holdings Inc., Onc.AI, Sonrai Analytics,
among others.
Artificial Intelligence In Clinical Trials Market
Artificial Intelligence In Clinical Trials Market Insights, Competitive Landscape, and Market Forecast – 2032
report delivers an in-depth understanding of the market trends, market drivers, market barriers, and key AI in clinical trials companies, including
TEMPUS, NetraMark, ConcertAI, AiCure, Medpace, Inc., ICON plc, Charles River Laboratories, Dassault Systèmes, Oracle, Certara, Cytel Inc., Phesi, DeepHealth, Unlearn.ai, Inc., H1, TrialX, Suvoda LLC, Risklick, Lokavant, Research Solutions
, among others.
Artificial Intelligence In Drug Discovery Market
Artificial Intelligence In Drug Discovery Market Insights, Competitive Landscape, and Market Forecast – 2032
report delivers an in-depth understanding of the market trends, market drivers, market barriers, and key AI in drug discovery companies, including
IBM Corporation, Numedii Inc, Deep Genomics, NVIDIA Corporation, Atomwise Inc, Cloud Pharmaceuticals Inc, Alphabet Inc (DeepMind), Insilico Medicine, BenevolentAI, Exscientia, Cyclia, Valo Health, Owkin Inc, Verge Genomics, BioSymetrics
, among others.
Artificial Intelligence in Drug Commercialization Market
Artificial Intelligence in Drug Commercialization Market Insight, Competitive Landscape, and Market Forecast – 2032
report delivers an in-depth understanding of market trends, market drivers, market barriers, and key AI in drug commercialization companies, including
EVERSANA, Lyfegen, Syneos Health, McKinsey & Company, ICON plc., Clarivate., Thermo Fisher Scientific Inc., Viseven, ZS Associates, Cloud Pharmaceuticals Inc.,
among others.
Artificial Intelligence in Precision Medicine Market
Artificial Intelligence in Precision Medicine Market Insight, Competitive Landscape, and Market Forecast – 2032
report delivers an in-depth understanding of market trends, market drivers, market barriers, and key AI in precision medicine companies, including
TEMPUS, GE HealthCare, Qure.ai, Envisionit Deep AI (Pty) Ltd., Avicenna.AI, Aignostics, Inc., Proscia Inc., Ultivue, Inc., Prenosis, Inc., IBEX, Cleerly, Inc., Paige AI, Inc., Densitas® Inc., Photocure ASA, iCAD, Inc., Eko Health, Inc., Owkin, Inc, Massive Bio, Deep Bio Inc., Atomwise Inc.,
among others.
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance.
Contact Us
Shruti Thakur
info@delveinsight.com
+14699457679
https://www.delveinsight.com/medical-devices
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability
for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this
article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
2025-05-14
·求实药社
就在上周,强生连放两枚重磅炸弹:其IL-23抑制剂Tremfya(古塞奇尤单抗)在中国拿下全球首个克罗恩病(CD)适应症,紧接着又在溃疡性结肠炎(UC)领域迅速获批,成为国内首个治疗UC的IL-23抑制剂。而在另一边,强生65亿美元(约467亿元人民币)重金收购双抗平台公司Proteologix,意图在IL-13/IL-17、IL-13/TSLP等炎症路径上拿下先机。从这一连串动作来看,强生的战略意图不言自明:用Tremfya续上Simponi、Stelara即将到期的火炬,同时加码下一代“双抗”,坐稳自免格局的C位。只是,这场“自免王座保卫战”背后,竞争比以往任何时候都更激烈,强生也并非没有后顾之忧。Tremfya,强生的“新王炸”Tremfya(guselkumab)是一款全人源IL-23p19单抗,最早在银屑病中脱颖而出,随后快速拓展至斑块型银屑病和银屑病关节炎。在UC与CD两个IBD核心适应症中,Tremfya在中国市场的获批节奏可能领先部分全球市场。以刚刚获批的UC为例,Tremfya在IIb/III期QUASAR研究中展现出优异数据:皮下注射维持治疗一年,临床缓解率达45%-50%,内镜缓解率超34%,均显著优于安慰剂组。最关键的是——患者在第一周即出现症状改善,远早于多数IL-23同类产品。而在CD领域,Tremfya今年2月便在中国实现了全球首次获批。也就是说,强生已在中国完成了“银屑病—关节炎—IBD”三大免疫主阵地的闭环。作为IL-23阵营中的扛旗者,Tremfya不只是一款药,更是强生“重构自免版图”的锚点。Stelara压力山大,Simponi逐步淡出为什么强生如此急切?答案很直接,Stelara专利即将到期。这款IL-12/23双靶点药物年销售额超100亿美元,是强生自免业务的营收支柱,但美国专利将在2025年失效,生物类似药大军已严阵以待;而另一款老将Simponi(TNF-α单抗)则已面临市场边缘化。强生显然不愿重蹈辉瑞、安进等“重磅品种断崖式下滑”的覆辙。它需要有充足的替代者,不仅覆盖适应症空白,还得匹配Stelara的商业表现——这也解释了Tremfya的多适应症“连发”、新路径加速推进。双抗背后的野心:对标下一代技术路线Proteologix之所以能引发强生65亿美元的抢购(几乎是无营收、无上市产品的早期平台),背后其实是一个路径压强的技术信号:双抗,将是下一代自免药物的新战场。此次收购包含两个核心临床前项目:PX128:IL-13/ TSLP 双抗,瞄准特应性皮炎(AD)和哮喘,即将启动Ⅰ期临床试验。PX130:IL-13/ IL-22 双抗,同样针对特应性皮炎,处于临床前开发阶段。PX128和PX130两款双抗,精准锚定Th2通路核心,覆盖特应性皮炎和哮喘。前者阻断上游激活因子TSLP和炎症核心IL-13,后者则切入皮肤屏障修复与局部免疫调控。靶点组合非简单叠加,而是路径闭环式协同,力图解决现有单抗疗效天花板,尤其针对难治性人群。对强生而言,这不仅是管线补强,更是对IL-13、IL-22、TSLP等热门靶点全面落地后的路径防御——要赢下未来,必须率先整合通路。对手没闲着:IL-23赛道越挤越狠Tremfya要打的仗,还远没结束。在IL-23单抗这一拥挤赛道上,强生面对的是礼来、艾伯维、诺华等老对手的正面交锋:Skyrizi(艾伯维):IL-23中最强竞品,2024年销售额超117亿美元,已获批银屑病、关节炎、CD;Mirikizumab(礼来):2023年FDA获批用于UC,定价略低,主打起效快;Netakimab(UCB):IL-17A/F 双靶向,银屑病表现强劲,间接挑战 IL-23 市场。尤其是Skyrizi,其在CD的表现极为强劲。研究显示,52周维持治疗后临床缓解率接近50%,且具有良好黏膜愈合能力,与 Tremfya 的 GALAXI 数据(56% 缓解率)几乎旗鼓相当,预示 IBD 市场的激烈博弈。双抗领域也不再是“空地”。赛诺菲的IL-13/TSLP双抗SAR443765已进入临床II期,艾伯维也有IL-17/IL-23融合蛋白正在开发。这意味着,即使强生“抢”下了平台,未来要胜出,还得靠真正的产品力和商业转化能力。临床、资本之外,强生也押注“长坡厚雪”除了并购、上市药的加速扩容,强生也在自免领域悄悄搭建起更长期的生态布局:与BenevolentAI等AI平台合作,推进炎症靶点的筛选与优化;通过JLABS扶持多家聚焦口服生物药、微生态疗法、递送系统的新兴企业;在Janssen研发内部,仍有超过10款早期自免药物处于IND或临床I期阶段,包括新型IL路径、小分子与细胞疗法。也就是说,强生并不满足于用Tremfya或双抗“接力”Stelara,而是在构建下一个“药王”的生态土壤。结语:续命,还是转身?从Tremfya的全球首发、UC/CD双线开花,到高溢价“抢走”双抗新星,强生自免板块确实亮出了不少“王炸”。但这些动作更像是一种主动求变:在Stelara的专利天花板逼近、IL-23竞争白热化、下一代路径尚未明朗的当口,强生选择了提前下场布局,而非等到退路变窄。能不能稳住这场转型,关键在两点:一是Tremfya能否在IBD市场对抗Skyrizi,二是Proteologix的双抗管线能否顺利进入临床、兑现技术溢价。强生这盘棋,刚刚落子,胜负未定。免责声明:文章内容仅供参考,不构成投资建议。投资者据此操作,风险自担, 关于对文中陈述、观点判断保持中立,不对所包含内容的准确性、可靠性或完整性提供任何明示或暗示的保证。请读者仅作参考,并请自行承担全部。联系我们I-RNA 2025展位火热预定中!扫码立即咨询电话:13816031174(同微信)赞助形式包括但不仅限于演讲席位、会场展位、会刊彩页、晚宴赞助、会议用品宣传等。点击此处“阅读全文”咨询更多!
并购专利到期生物类似药临床3期
2025-04-29
·智药邦
2025年4月17日,专注于调研生物医药行业趋势和创新的BioPharmaTrend发布文章:Beyond Legacy Tools: Defining Modern AI Drug Discovery for 2025 and Beyond。以下是作者的核心观点:AIDD的核心理念并非通过使用更优模型或高级机器学习等技术来改进现有药物研发流程。虽然业内大多数公司目前确实专注于此类局部优化,但更好的筛选模型或分子对接技术只能带来边际效益提升,无法根本解决药物研发的核心痛点:从假说到临床结果的转化率低下,以及因意外毒性或疗效不佳导致的临床高失败率。AIDD的真正创新性与战略雄心在于:将现有主流药物研发范式重构为全新模式。作者建议将其命名为"整体药物开发(HDD)"。该模式从患者全维度数据建模出发(涵盖生物标本、分析样本、电子健康档案等现实世界数据),整合所有可用临床前数据和经验,在分子层面建立科学假说。继而逆推实施:沿着新构建的科学假说路径,通过药物设计与开发回归患者治疗场景,最终提升临床成功率。本报告目录引言:新框架AI药物发现:强调整体性AI药物发现:以构建软件为核心数据获取为王验证对AI药物发现平台至关重要2025年及以后的AI药物发现愿景引言:新框架2025年,对于新兴的一类由人工智能驱动的药物发现公司(以下简称“AIDD公司”),似乎仍缺乏一个完善可靠的定义。 本报告的目的是提出一个用于对AIDD公司进行分类的定性框架,该框架综合了定义这一领域领先企业的四个关键属性: 1. 在生物学研究中侧重于整体论还是还原论;2. 创建强大的人工智能平台(软件);3. 数据获取的优先级;4. 技术验证(通过展现出发现新靶点的能力、快速发现和开发临床级候选药物的能力、平台合作的过往记录、科学出版物、专利等方面来体现)。我们将在下文深入探讨这一框架,但简而言之,其核心要点如下:核心价值点可归结为三个问题:计算平台是否足够扩展性强且稳健,能够通过改善研发流程、协作模式和日常决策来提升生产力?能否对生物学进行深度和广度的建模,以支持超越传统方法的科学决策?能否以可重复、稳定的标准化方式在全研发流程中实现上述两点?AI药物发现:强调整体性在我们探索新提出的理论框架时,一个关键区别逐渐显现:在当下人工智能驱动的技术背景下,我们在尝试建模和计算表征的对象,与早期计算工具通常处理的内容存在本质差异。理解这种转变的一个有效切入点,是思考传统软件与现代AI平台之间的概念鸿沟。那些开发于数十年前的传统软件至今仍在药物发现中承担特定任务,而现代AI支持的平台越来越被定位为端到端解决方案。尽管两类工具都发挥着重要作用,但它们的基础理念却大相径庭。简而言之,"传统"的化学信息学与生物信息学采用人工驱动的研究范式:化学信息学通过预定义的化学描述符(如分子量或logP值),结合统计方法和部分机器学习模型,应用在QSAR建模和分子对接等任务中;而生物信息学则运用包括降维技术在内的统计方法,来分析复杂的生物数据集(如基因组学、蛋白质组学),进而挖掘潜在药物靶点。这些方法以假设为导向,采用模块化设计,适用于规模较小、结构良好的数据集。从理念层面看,传统计算系统和简单机器学习方法在"生物学还原论"框架下表现出色(即使在今天其价值依然显著)。典型的还原论研究案例当属基于结构的药物发现,该范式主张通过调控特定蛋白质来破解药物研发难题(这一思路在某些情况下确实有效)。其对应的计算建模主要聚焦于单一任务场景,例如将配体置入蛋白质结合腔(分子对接),或者通过计算方法识别针对给定靶点的新型化学结构(基于配体的虚拟筛选)。Laozhengzz的“基于结构的药物设计流程图”,通过维基共享资源(CC by-SA 3.0)与之形成鲜明对比的是,当今最前沿的人工智能驱动型药物发现公司正在将研究提升至系统生物学层面。这类公司采用不依赖预设假设的研发范式,通过基于深度学习的分析系统整合多模态数据(包括表型数据、组学数据、患者数据、化学结构、文本及影像等),最终构建出复杂而完善的生物学表征体系(如"知识图谱")。例如,美国波士顿英矽智能公司Pharma.AI计算平台的科学基础源于基于策略梯度的强化学习(RL)与生成模型的新型融合,由此实现多目标优化,以平衡效力、毒性和新颖性等参数。该公司表示,其靶点识别模块PandaOmics整合了来自超1,000万份生物样本(包括RNA测序和蛋白质组学数据)和4,000万份文档(如专利与临床试验)的1.9万亿个数据点,通过自然语言处理和机器学习技术发掘并优先排序新型治疗靶点。化学生成模块Chemistry42应用深度学习技术(包括生成对抗网络和强化学习)设计具有优化结合亲和力、代谢稳定性及生物利用度的新型类药分子。在临床开发阶段,临床试验预测系统inClinico利用历史与进行中的试验数据预测结果,为患者筛选和终点优化提供洞见。在算法层面,Pharma.AI采用先进的奖励塑造技术,可根据特定靶点特征或多药理学靶点对生成分子进行微调。此外,英矽智能重点应用知识图谱嵌入技术,将基因-疾病、基因-化合物及化合物-靶点相互作用等生物关系编码到向量空间中。这些嵌入特征通过受Transformer模型启发的注意力神经网络架构增强,聚焦生物学相关子图结构,优化靶点识别与生物标志物发现的假说生成。该平台实施持续主动学习与迭代反馈机制,通过新实验数据(包括生化检测、表型筛选和体内验证)对模型进行再训练,快速剔除次优候选分子并加强先导化合物发现,从而加速"设计-合成-测试-分析"(DMTA)循环。此外,平台的多模态数据融合技术整合了来自文献、专利和临床试验的文本信息,以及多组学洞见与化合物库资源。为此,自然语言处理(NLP)模型从文本源中提取相关生物学背景与副作用注释,并辅以表型筛选数据,实现对药物发现过程的全方位把控。读者可通过近期发表的论文《A small-molecule TNIK inhibitor targets fibrosis in preclinical and clinical models》深入了解Pharma.AI平台的部分核心技术。另一个可归类为人工智能药物发现方法的典型案例是Recursion的OS平台。Recursion OS是一个整合多种技术的纵向平台,使该公司能够利用约65PB的专有数据,绘制并探索数万亿级生物、化学及以患者为中心建立的关系网络。根据Recursion官方说明,该平台将湿实验室自主生成或精选合作方提供的"真实世界"数据,与公司内部构建的人工智能计算模型集合"世界模型"相结合。目前,其规模化产生的湿实验室生物学、化学及面向患者的实验数据,为干实验室计算工具提供燃料,用以识别、验证和转化治疗发现洞见,这些成果又可返回湿实验室进行验证。Recursion OS由BioHive-2超级计算机驱动,该公司宣称这是目前生物制药企业全资拥有并运营的最快速超级计算系统。尽管在模型架构和工作流程上与英矽智能存在差异,但Recursion同样专注于核心目标:构建完整的生物学数字化表征体系,以此挖掘药物开发的关键洞见。递归操作系统平台的概念化表示Recursion OS的关键模型包括Phenom-2,这是一个拥有19亿参数的ViT-G/8掩码自动编码器(MAE),在80亿张显微图像上进行训练,据该公司称,在基因扰动可分性方面实现了60%的提升。MolPhenix是2024年神经信息处理系统大会(NeurIPS)最佳论文奖得主,它能预测分子-表型效应,相比基线模型有显著提升。MolGPS是一个拥有30亿参数的模型,在分子性质预测方面表现出色,并且整合了专有的表型组学数据,在22项药物吸收、分布、代谢、排泄和毒性(ADMET)任务中的12项上超越了基准模型。MolE在8.42亿个分子图上进行训练,在22项ADMET任务中的10项中处于领先地位。Recursion OS的一个有趣组件是一个知识图谱工具,该工具通过生物学和药物发现领域一系列复杂的相关主题视角,对Recursion OS发现的潜在信号进行评估,这些主题包括全球趋势评分、蛋白质口袋和结构、竞争格局以及临床试验等。这个知识图谱使研究人员能够进行“靶点解析”,即识别和验证小分子表型反应的分子靶点,从而将数百种可能性缩小到最佳靶点机会。一个较新的例子来自总部位于加利福尼亚州的Iambic Therapeutics公司,该公司成立于2019年。Iambic的团队开发了一个药物发现平台,该平台将三个专门的AI系统——Magnet、NeuralPLexer和Enchant整合到一个统一的流程中,从计算层面涵盖了分子设计、结构预测和临床性质推断等方面。Magnet通过利用受Iambic自动化化学基础设施约束的反应感知生成模型,产生可合成的小分子。这些分子随后被输入NeuralPLexer——这是一种基于多尺度扩散的生成模型,仅需以蛋白质序列和配体图为输入,即可直接预测蛋白质-配体复合体中原子的配体诱导构象变化。所得结构复合体能够反映靶向参与度和结合特异性。最终,Enchant采用经过多样化且噪声丰富的临床前数据集训练的多模态变换器架构,通过迁移学习预测人体药代动力学和其他临床效果,即便在临床数据有限的情况下仍能保持高预测精度。该架构实现了迭代式的模型驱动工作流程:候选分子在合成前即可通过计算机全流程完成设计、结构评估和临床优先级排序。最后值得注意的是神经退行性疾病领域的Verge Genomics案例。该公司开发的CONVERGE®平台是一套端到端闭环机器学习系统,将大规模人类来源的生物数据与预测建模相整合。该系统核心利用高维度多模态数据集,包括:超过60TB的人类基因表达和推断的基因关联数据、数千项基因扰动和ChIP-seq研究、数百万个蛋白质互作,以及来自渐冻症、帕金森和额颞叶痴呆等疾病患者的直接人类临床样本。这些数据用于训练机器学习模型,以识别和优先考虑具有更高转化相关性的药物靶点,避免依赖那些难以模拟人类生物学的动物或人工细胞模型。通过Verge自有的湿实验室设施,对这些模型的预测结果进行内部实验验证,形成反馈循环,持续优化生物学假设和模型性能。这种将患者来源的组织数据、机制基因组学与计算靶点优先级排序相结合的方法,旨在无需进行盲目的大规模筛选即可识别临床可行的候选药物。Verge自主开发的临床化合物完全通过CONVERGE®平台在四年内完成开发,包括靶点发现阶段。从概念上讲,"AI药物发现"(相较于"传统"计算系统)指的是现代化的计算技术架构——通常是多模态集成系统。这类系统能够对生物学进行整体建模,同时处理各类形式和规模的数据(分子、表型和临床数据,包括化学组学、文本、图像(如细胞染色)、电子健康记录等),或其大部分组合。生成式AI现代AI药物发现与传统计算工具的另一个关键区别在于生成能力。尽管英矽智能等公司在2016年率先使用生成对抗网络(GANs)进行生成化学研究——通过利用GANs建模复杂分子分布并提出新颖化学结构的能力;但自2017年Transformer架构和注意力机制的引入(特别是BERT和GPT等模型的问世),我们认为这标志着生成模型跨领域的范式转变。在具有里程碑意义的论文《Attention Is All You Need》发表后,我们将2017年视为生成式AI(涵盖化学和生物学领域)的奠基之年。这些最初由Google开创、后来由OpenAI、Anthropic、Mistral AI等公司发展的架构,展示了处理序列数据中长距离依赖关系的无与伦比扩展能力。通过在超大规模文本语料库上预训练(模型参数达数千亿甚至上万亿量级),并运用自注意力机制动态分配输入关系的权重,Transformer架构使GPT-3、GPT-4等大规模生成模型能够输出高度连贯且上下文精确的内容。诚然,"幻觉生成"仍是一个突出问题,但范式转变已至为深刻。该领域的先驱商业产品主要包括:面向文本到文本生成的ChatGPT,专注文本到图像生成的Midjourney,以及众多致力于文本到视频、文本到音乐转换的创新应用。实际可用的Transformer架构和大语言模型的出现,催化了计算化学与生物学领域对所谓"基础模型"的研发竞赛。简而言之,我们可用一个普适性框架来厘清传统计算机辅助药物设计(CADD)与现代人工智能驱动药物发现(AIDD)的本质关联:在探讨过"AI药物发现"试图建模的对象(整体生物学vs主流"还原论")以及实现这一目标所需模型的特性后,让我们转向另一个关键维度——作为软件产品的AI平台"成熟度"。AI药物发现:以构建软件为核心头部AI制药平台与"伪AI"公司最显著的区别,就在于前者展现出明确的软件工程化导向。这个本应显而易见的事实,却在许多行业分析师、科技媒体观察家的评论中被长期忽视。我们应当要求AIDD公司必须能够展示其拥有一套稳健、自主的软件平台,该平台需支持关键功能——包括面向数据输入和参数调整的用户友好界面(GUI),以及可配置的机器学习模块(涵盖算法选择、超参调整和模型性能可视化等)。此类平台需将标准化数据输入管道(如组学数据、小分子化合物库或临床元数据处理)与支持动态模型训练、验证及迭代优化(如主动学习、强化学习循环)的后端组件相结合。此外,详尽的应用程序接口(API)文档对于与外部工具的互操作性至关重要,确保终端用户能实现工作流自动化并在软件组件间无缝交换数据。同时,一套完善的端到端解决方案应内置安全性保障、数据完整性措施(如版本控制、审计追踪、加密)及灵活的部署选项(本地或云端),以满足不同组织的需求。对于符合新兴框架所定义的“AI药物发现”平台,用户通常能够查看甚至试用其软件演示版。在某些案例中,如英矽智能、Schrodinger、OWKIN、Iktos、CytoReason、BenchSci等公司,用户可获取许可并用于内部项目。然而,我们发现绝大多数宣称从事AI驱动业务的公司并未公开其软件特性或提供实时演示的相关信息。在AIDD领域,企业软件平台的成熟度绝非细枝末节——它是根基所在。这是因为现尚无任何AI解决方案能仅凭一键操作就独立产出临床级别的治疗药物。尽管技术取得了显著进步,当今的AI系统主要扮演着智能副驾驶的角色——它们是辅助而非取代人类科学家专业能力的工具。鉴于这种辅助属性,AI系统的真正价值在于其如何无缝融入企业内部工作流。若要对研发效率和创新质量产生实质影响,软件不能仅局限于模型或接口的集合——它必须是一个成熟、可互操作且能在组织中规模化扩展的平台。缺乏此等软件成熟度,AI在药物发现中的承诺仍将主要停留在理论层面。AI药物发现公司虽横跨软件与生命科学两大领域,但其估值与对标体系仍以医药行业为核心。当前的评估重点聚焦于临床管线进展、监管里程碑及湿实验验证,而往往忽视诸如模型准确性、算法可扩展性、数据所有权和计算效率等软件驱动的关键指标。鉴于许多AIDD企业通过AI赋能平台、预测分析及专有数据集创造价值,其商业潜力也应采用软件行业方法论进行评估——例如AI-as-a-service模式收入、专有算法知识产权价值及云端可扩展性。更全面的对标框架应整合软件与医药行业标准,以更精准捕捉AIDD公司多元化的价值主张。数据获取为王我们预见2025年数据生成、整合与应用分析领域将加速发展——尤其在以下技术:新一代测序(NGS)、先进蛋白质组学、质谱分析、冷冻电镜、芯片器官系统及机器人化实验室。这些技术是构建更丰富、更全面的药物发现与转化研究数据库的基石。此背景下,Tempus等公司代表了一类更广泛的数据基础设施供应商。Tempus专注于临床与分子数据的聚合与结构化,并开发支持数据可及性与临床决策的软件系统。其平台被医疗机构及生命科学组织用于指导诊断、治疗选择及研究。随着生物医学数据的量与复杂性持续攀升,此类平台或将成为整合真实世界与实验数据集以支持AI驱动发现工作流的核心。早期如英矽智能、BPGbio、Recursion及近期崭露头角的NOETIK等AIDD公司,已将数据获取作为资产价值基石进行持续投资。例如,英矽智能于2022年12月推出的“第六代”智能机器人药物发现实验室,将AI决策系统与全自动化机器人模块相结合,覆盖靶点发现、化合物筛选、精准药物开发及转化研究全流程。英矽智能在中国苏州的第六代机器人实验室根据公司介绍,其Pharma.AI平台通过与六大功能模块的结合——涵盖自动化细胞培养、高通量筛选、下一代测序(NGS)和高内涵成像——实验室构建了闭环验证系统,用于新靶点验证、先导化合物优化,并通过高质量生物数据训练优化AI模型。另外的典型案例之一是Recursion基于65+PB医药多组学数据构筑护城河:包含表型组学、转录组学、蛋白质组学、ADME、活体验证组学、基因组学以及患者数据等多元数据集。通过每周进行220万次高通量实验(CRISPR-Cas9基因编辑技术+Brightfield成像技术)累积产生约36PB内部数据,形成生物医药领域最大的数据集之一,并运用AI模型进行生物解析。据公司独家访谈披露,其处理了22PB转录组学数据,并整合了来自Helix和Tempus的20PB患者数据,涵盖数十万案例的全基因组与外显子组测序。在细胞产业化方面,针对与罗氏/基因泰克合作开展的40个神经科学和肿瘤学项目,公司年产1万亿个hiPSC诱导神经元细胞构建"神经图谱"(Neuromap)。另一个例子是CytoReason通过整合海量公共/私有数据集(批量+单细胞转录组学、蛋白质组学、临床数据)构建统一AI疾病模型平台。该平台运用先进机器学习算法,在细胞分子层面解构治疗方案、患者群体与疾病机制,支持跨疾病/跨组织综合分析。第三个典例是Berg Health(现BPGbio)通过建立包含10万+临床注释人类生物样本库(生物液体/组织标本),驱动AI药物发现平台。公司对样本进行基因组学、蛋白质组学、代谢组学和脂质组学的多组学分析,通过NAi Interrogative Biology®平台运用贝叶斯人工智能算法解析高维数据挖掘生物标志物与治疗靶点,历史性促成多项成功临床试验。最新案例则是NOETIK通过策源临床级人类肿瘤样本构筑标准化生物样本库:严格质控缺血时间、坏死率、样本年龄等参数,并通过病理学家复核筛选。通过空间转录组学、全外显子测序和定制化蛋白面板建立多维数据集解析肿瘤-免疫微环境互作,采用空间随机排列组织微阵列技术降低切片假象干扰。NOETIK的数据基础这段庞大的数据流水线,结合其正在申请专利的工艺流程,使其能够创建高质量的自我监督训练数据集,为其人工智能引擎OCTO提供动力。OCTO专门用于肿瘤生物机制建模和预测患者特异性治疗反应。AI药物发现平台的验证至关重要最终,AIDD领域的核心可信度指标在于平台验证,通常需要展示可执行成果及不同应用场景下的可复现性。平台验证可通过以下方式的组合实现:1.推进内部新药研发管线:利用AI引擎辅助研发团队进行先导分子的发现、设计与优化,推动其完成临床前研究乃至部分进入临床开发阶段;2.与知名药企或生物技术机构合作:通过第三方机构使用专有数据集测试AI平台的预测能力与生成能力,此时公开里程碑成果的记录至关重要;3.通过公开软件演示/经同行评审期刊发表的概念验证研究及专利;4.定期在权威期刊发布AIDD实际应用案例。下方表格展示了AI制药领域中多家标杆企业的历史研发管线增长态势,包括BenevolentAI、Healx、英矽智能、Schrodinger、Relay Therapeutics、Recursion(2024年完成对Exscientia的收购)、Valo Health、Verge Genomics等企业。注:表格中上市公司数据透明度通常更高。对上述表格数据和信息图表进行解读可见,英矽智能在过去五年展现出显著的产品管线增长。具体而言,这家公司已启动了针对多种适应症的31个治疗项目:2021年提名了22个临床前候选药物,2022年又新增9个,其中共有10条管线获得新药临床试验申请(IND)批准。目前其治疗特发性肺纤维化(IPF)的头部项目,从概念验证到一期临床试验仅用了不到30个月的时间,当前正在美国和中国同步开展2期临床试验,另还有五个项目处于1期阶段。此外,多个临床资产已通过对外授权或与第三方合作开发模式实现价值,包括近期公布的里程碑付款案例。虽然这些成就突显出研发效率的大幅提升,但关键问题在于:这些成果是否确凿证明了AI技术——而非传统研发架构与合作伙伴关系——才是实现快速扩张的核心驱动力?Schrodinger的平台亦呈现出类似现象。尽管未被明确冠以"AI"标签,但其软件能力成功支撑了可观的管线开发规模。诚然,此类技术能通过加速靶点识别、优化先导化合物等方式提升决策效率,但这并不意味着能够"打印"成功分子。要判断人工智能的真实贡献,需评估这些平台能否在统计学意义上显著缩短药物发现周期,或提升研发成功率。更有说服力的验证维度或许是考察有多少第三方机构真正采纳并有效运用这些工具进行药物开发,从而形成外部反馈与现实效能的衡量标尺。除AI企业的管线数量外,观察头部玩家选择的靶点新颖性亦颇具启示:新药研发速度与成本近年来一些由人工智能驱动的药物发现公司所报道的数据显示(从项目启动到新药临床试验申报阶段),其临床前候选药物提名所需时间似乎比已知行业平均周期有所缩短。例如,英矽智能、Recursion和Exscientia等企业成功将原先行业标准的2.5-4年(即40-50个月)药物发现阶段,在某些案例中压缩至9-18个月。根据最新发布的行业基准值:英矽智能每个项目平均耗时12-18个月,仅需测试60-200个分子;Recursion能在18个月内推进候选药物,每个项目测试分子数低于200个。而与Recursion合并后的Exscientia宣称,其研发周期从传统模式的4-5年缩短至12-18个月,对150-250个分子进行筛选——这与传统方法形成鲜明对比,后者每项目有时需要测试3,000-5,000个分子。尽管这些看似加速的时间线尚未完全转化为临床成功。虽然一些由AI开发的药物已进入进行中的临床试验(例如来自英矽智能、Iambic和Recursion的项目),但也有相当数量因临床试验失败或战略调整而终止的案例。虽然AI计算工具可以更快预测有前景的候选药物(根据多方声明),但这并不能保证这些药物具备临床可行性、有效性或安全性。AI驱动项目筛选分子数量的减少也可能带来风险——过度收窄搜索范围可能导致关键的缺陷在后期临床开发中才暴露出来。除了研发周期,成本也是重要考量因素。虽然本项研究不涉及这类项目的成本结构,但英矽智能曾声称其部分AI设计的候选药物发现成本仅为"传统"项目的10%左右(该声明未经我们专门验证)。对AIDD投资者及利益相关方的务实建议:突破候选数量表象:单纯统计管道资产数量无法体现AI平台可能带来的增值价值。更快速的研发启动周期或更精准的淘汰率可能是更具说服力的指标。评估决策效率提升:聚焦AI显著缩短研发流程的环节——例如加速先导化合物优化阶段、改进靶点验证效率,或支持更高效的临床试验方案设计。审视外部验证成果:寻找第三方支持的生产力提升证据,例如合作公告、达成的里程碑事件或持续软件许可协议。开放授权或商业化的工具更便于进行竞争对标。考量环境影响因素:需认识到企业战略、资金实力和现有研发基础设施往往对管线产出起决定性作用。在未分析这些并行因素的情况下,难以单独评估AI的实际贡献。事实上反过来说:几乎不可能精确计算AI算法对实际药物开发过程产生的真实影响。2025年及以后的AI药物发现愿景通过近十年来对众多声称采用AI技术的药物研发公司的追踪观察(其间经历了技术进步、市场炒作和现实检验——例如所谓AI设计候选药物的临床试验失败),当前正是定义AI药物发现目标与方法的关键时刻,需要正视绝大多数企业仍处于探索阶段这一现实。事实上,AIDD的核心理念并非通过使用更优模型或高级机器学习等技术来改进现有药物研发流程(如基于结构的药物发现或虚拟筛选)。虽然业内大多数公司目前确实专注于此类局部优化,但更好的筛选模型或分子对接技术只能带来边际效益提升,无法根本解决药物研发的核心痛点:从假说到临床结果的转化率低下,以及因意外毒性或疗效不佳(有时源自欠佳的患者亚群选择)导致的临床高失败率。AIDD的真正创新性与战略雄心在于:将现有主流药物研发范式重构为全新模式。我们建议将其命名为"整体药物开发(Holistic Drug Development, HDD)"。该模式从患者全维度数据建模出发(涵盖生物标本、分析样本、电子健康档案等现实世界数据),整合所有可用临床前数据和经验,在分子层面建立科学假说。继而逆推实施:沿着新构建的科学假说路径,通过药物设计与开发回归患者治疗场景,最终提升临床成功率。尽管真正实现这一愿景仍需经年累月的努力,但已有多家创新企业正在为未来的工业化研发体系拼筑关键模块。时间将验证AIDD是否是实现HDD愿景的更优路径。对此,我们保持审慎乐观态度。参考资料:https://www.biopharmatrend.com/ai-drug-discovery-pipeline-2024/--------- End ---------感兴趣的读者,可以添加小邦微信加入读者实名讨论微信群。添加时请主动注明姓名-企业-职位/岗位或姓名-学校-职务/研究方向。
100 项与 BenevolentAI Ltd. 相关的药物交易
登录后查看更多信息
100 项与 BenevolentAI Ltd. 相关的转化医学
登录后查看更多信息
组织架构
使用我们的机构树数据加速您的研究。
登录
或

管线布局
2026年02月28日管线快照
管线布局中药物为当前组织机构及其子机构作为药物机构进行统计,早期临床1期并入临床1期,临床1/2期并入临床2期,临床2/3期并入临床3期
药物发现
3
2
临床前
临床1期
1
14
其他
登录后查看更多信息
当前项目
| 药物(靶点) | 适应症 | 全球最高研发状态 |
|---|---|---|
BEN-8744 ( PDE10A ) | 溃疡性结肠炎 更多 | 临床前 |
BEN-34712 ( RARα x RARβ2 ) | 肌萎缩侧索硬化 更多 | 临床前 |
BEN-28010 ( Chk1 ) | 胶质母细胞瘤 更多 | 临床前 |
Fibrosis therapy(BenevolentAI Ltd) | 纤维化 更多 | 药物发现 |
Systemic Lupus Erythematosus (BenevolentAI) | 系统性红斑狼疮 更多 | 药物发现 |
登录后查看更多信息
药物交易
使用我们的药物交易数据加速您的研究。
登录
或

转化医学
使用我们的转化医学数据加速您的研究。
登录
或

营收
使用 Synapse 探索超过 36 万个组织的财务状况。
登录
或

科研基金(NIH)
访问超过 200 万项资助和基金信息,以提升您的研究之旅。
登录
或

投资
深入了解从初创企业到成熟企业的最新公司投资动态。
登录
或

融资
发掘融资趋势以验证和推进您的投资机会。
登录
或

生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用