预约演示
更新于:2025-05-07
MAP3K14
更新于:2025-05-07
基本信息
别名 FTDCR1B、HS、HSNIK + [6] |
简介 Lymphotoxin beta-activated kinase which seems to be exclusively involved in the activation of NF-kappa-B and its transcriptional activity. Phosphorylates CHUK/IKKA, thereby promoting proteolytic processing of NFKB2/P100, which leads to NF-kappa-B activation via the non-canonical pathway (PubMed:25406581, PubMed:29230214). Has an essential role in the non-canonical NF-kappa-B signaling that regulates genes encoding molecules involved in B-cell survival, lymphoid organogenesis, and immune response (PubMed:25406581). Could act in a receptor-selective manner. |
关联
10
项与 MAP3K14 相关的药物作用机制 MAP3K14 抑制剂 [+1] |
在研机构 |
原研机构 |
在研适应症 |
非在研适应症- |
最高研发阶段临床前 |
首次获批国家/地区- |
首次获批日期1800-01-20 |
靶点 |
作用机制 MAP3K14 抑制剂 |
在研适应症 |
非在研适应症- |
最高研发阶段药物发现 |
首次获批国家/地区- |
首次获批日期1800-01-20 |
100 项与 MAP3K14 相关的临床结果
登录后查看更多信息
100 项与 MAP3K14 相关的转化医学
登录后查看更多信息
0 项与 MAP3K14 相关的专利(医药)
登录后查看更多信息
2,322
项与 MAP3K14 相关的文献(医药)2025-08-01·Bioresource Technology
NaCl as an excellent trigger-induced biodiesel production and phenol-containing wastewater treatment in a novel salt-tolerant microalgae Ankistrodesmus sp. ACC
Article
作者: Hu, Gen ; Wang, Yanlin ; Yousif Abdellah, Yousif Abdelrahman ; Elsheikh, Elsiddig A E ; Yang, Jinzhengyu ; An, Xuejiao ; Wang, Lijing
2025-06-01·Fish & Shellfish Immunology
Molecular evolution and functional characterization of PKC-α-like in Lamprey
Article
作者: Sun, Feng ; Zhang, Xingzhu ; Gou, Meng ; Lu, Jiali ; Xu, Yang ; Zhao, Huan ; Liu, Zhulin ; Gao, Zhanfeng ; Li, Xue ; Ren, Kaixia ; Li, Qingwei ; Han, Yinglun
2025-05-01·European Journal of Medicinal Chemistry
The discovery of novel N-heterocyclic-based AKT inhibitors with potential efficacy against prostate cancer
Article
作者: Gong, Kexin ; Jiao, Zhihao ; Yang, Dezhi ; Huang, Yongmi ; Sun, Jinxiao ; Yu, Shangzhe ; Zhao, Guisen ; Liu, Yiru
2
项与 MAP3K14 相关的新闻(医药)2024-11-19
BEIJING, Nov. 19, 2024 /PRNewswire/ -- With the support of the RiDYMO® platform, we are able to comprehensively explore various potential targets in drug development, including protein-protein interactions, kinases, GPCRs, ion channels, and transcription factors.
Continue Reading
Figure: Screening process for novel scaffold lead compound
Figure A: Optimization and FEP prediction for R1, R2, and R3, with dark gray areas indicating a 1 kcal error range and light gray areas representing a 2 kcal error range; B: Overall molecular optimization process based on the DP101 scaffold
Figure: Antitumor effects of DP226 on HCT116 xenograft model
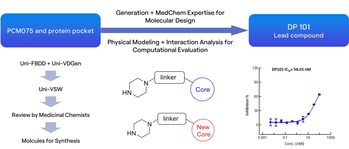
Polo-like kinase 1 (PLK1) is a highly conserved serine/threonine protein kinase that plays a crucial role during cellular mitosis[1]. It is overexpressed in various malignancies, including colorectal cancer[2], pancreatic cancer[3], and breast cancer, and its high expression is associated with poor prognosis. Consequently, PLK1 has emerged as a promising target in cancer drug development, with numerous candidates currently in the pipeline worldwide. Among these, Cardiff Oncology's onvansertib (PCM-075) has shown the most significant progress, displaying encouraging efficacy results in clinical treatment for patients with metastatic colorectal cancer (mCRC).
The RiDYMO® platform, a next-generation computational drug design platform developed by DP Technology, based on AI for Science, provides robust end-to-end support for hit discovery and optimization by integrating multiple artificial intelligence techniques, physical algorithms, and experimental validation. In this study, we referred to the co-crystal complex of PLK1 and PCM075 (2YAC) and utilized the RiDYMO platform's comprehensive capabilities to synthesize and test under 100 compounds, leading to the identification of preclinical candidate compounds with potential clinical value and significantly accelerating the drug development process.
We first conducted an in-depth analysis of the interactions between PCM-075 and the PLK1 protein. Preserving key interactions, we applied molecular generation, evaluation, and screening techniques to obtain a novel scaffold, the lead compound DP101, which exhibited an activity of 56 nM.
Next, during the optimization process based on the structure of DP101, we modified several sites on the scaffold, including hydrogen bonding networks (R3) and hydrophobic group encapsulation zones (R1 & R2). Experimental data showed a high consistency between computational predictions and experimental results, demonstrating the platform's practicality in optimizing kinase inhibitors.
Leveraging the RiDYMO® platform's activity prediction ranking and physicochemical property forecasting, we underwent multiple rounds of testing and optimization, ultimately obtaining several compounds with enhanced in vitro activity and pharmacokinetic properties. Notably, the activity of DP226 (0.19 nM) improved nearly 300-fold compared to DP101, surpassing PCM075, with an oral bioavailability (F = 76.8%) significantly higher than that of PCM075 (F = 24%). At low doses, DP226 exhibited superior anti-tumor effects compared to PCM075, and in combination with bevacizumab, it led to tumor regression, positioning it as a potential PCC molecule for further studies.
Dr. Dongdong Li, Director of Medicinal Chemistry at DP Technology and Project Leader, stated, "With the support of the RiDYMO® platform, we are able to comprehensively explore various potential targets in drug development, including protein-protein interactions, kinases, GPCRs, ion channels, and transcription factors. The use of the RiDYMO® platform for activity predictions, rankings, and experimental validations significantly enhances the efficiency of activity optimization, effectively shortening optimization cycles and accelerating the overall project development timeline. The integrated capabilities of the RiDYMO® platform hold promise for providing high-efficiency support in early-stage drug discovery. As a potential PCC molecule, we look forward to collaborating with experienced pharmaceutical companies in this field to advance this project to the next milestone."
About the RiDYMO® Hit Discovery and Optimization Platform
RiDYMO® is a state-of-the-art hit discovery and optimization platform developed by DP Technology, leveraging the principles of AI for Science. It employs the proprietary Hermite® computational drug design software to elucidate the dynamics of "undruggable" targets and explores a broader chemical space encompassing small molecules, macrocycles, and cyclic peptides. By integrating advanced artificial intelligence, physical algorithms, and high-throughput experimentation, the platform excels in designing oral macrocyclic compounds and rapidly delivers innovative drug candidates.
As one of its core algorithms, Reinforced Dynamics (RiD)[4] has a significant advantage in the sampling efficiency of molecular dynamics simulation. By fully leveraging the high-dimensional representation capabilities of neural networks, RiD can efficiently capture dynamic conformational changes in complicated biomolecular systems. Previously, the core RiD algorithm of the platform was published in Nature Computational Science. The study demonstrated that RiD could achieve a more comprehensive free energy surface within 1.86 μs, compared to 100 μs required by traditional MD methods, representing nearly a hundredfold increase in efficiency.
RiDYMO® has been successfully employed in various projects, including the development of c-Myc small molecules, GPX4 small molecules, and β-catenin cyclic peptides, demonstrating its strong potential in innovative drug discovery.
For more information, please visit our website.
().
About Hermite
Hermite® is a next-generation computational drug design platform developed by DP Technology, powered by AI for Science, to provide a comprehensive solution for drug design. Hermite® integrates industry-leading tools such as the Free Energy Perturbation module, Uni-FEP, and the ultra-high-throughput virtual screening tool, Uni-VSW, supporting key stages of drug discovery, from protein structure prediction and target validation, to hit discovery and lead optimization.
The platform offers an interactive, web-based molecular visualization experience, with detailed management of projects, teams, and data. It features full compliance certification, multi-tier security measures, and supports both cloud-based and private deployment options.
Hermite® is trusted by over 60% of leading pharmaceutical companies in China, applied across more than 50 drug pipelines. To date, industry users have performed over 200,000 Uni-FEP calculations on the Hermite® platform.
For more information, please visit:
About DP Technology
At DP Technology, we're at the forefront of integrating artificial intelligence into scientific research and industrial R&D. Our "AI for Science" initiative is redefining how we tackle complex scientific challenges, making groundbreaking discoveries more accessible and actionable.
We've developed the "DP Particle Universe," a suite of advanced pre-trained models that seamlessly connect cutting-edge research with real-world industrial applications. Our software suite includes: Bohrium® Scientific Computing Space Station, Hermite® Computational Drug Design Platform, RiDYMO® Hit Discovery Platform, and Piloteye® Battery Design Automation Platform. Together, these platforms form a robust foundation for industrial innovation and an open ecosystem for AI in science, fostering advancements in key areas such as drug discovery, energy, materials science and information technology.
SOURCE DP Technology
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
440k+
Newsrooms &
Influencers
9k+
Digital Media
Outlets
270k+
Journalists
Opted In
GET STARTED
蛋白降解靶向嵌合体
2021-10-12
Agreement Expands on the Companies' Collaboration for the Preclinical Development of Potential Anti-Cancer Therapies
[12-October-2021]
TORRANCE, Calif., Oct. 12, 2021 /PRNewswire/ -- Emmaus Life Sciences (Formerly Known As Emmaus Medical, Inc.) (OTCQX: EMMA), a commercial-stage biopharmaceutical company and leader in the treatment of sickle cell disease, today announced the signing of an agreement with Kainos Medicines (Kainos), granting Emmaus an exclusive license to patent rights, know-how and other intellectual property relating to Kainos' novel IRAK4 inhibitor, referred to as KM10544, for the treatment of cancers including leukemia, lymphoma and solid tumors. The license agreement expands upon the existing research and development collaboration agreement between the parties. Financial details of the agreement were not disclosed.
Emmaus intends to advance the licensed technology to complete in-vivo studies to determine disease selection for KM10544 which, if successful, would be followed by Investigational New Drug enabling studies. Emmaus also plans to seek Orphan Drug Designation for relevant clinical indications.
"Obtaining the exclusive license to the intellectual property surrounding Kainos' IRAK4 inhibitor is an important milestone as we expand our pipeline with a novel potential treatment option for some of the most resilient lymphomas in the world," stated Yutaka Niihara, M.D., M.P.H., Chairman and Chief Executive Officer of Emmaus. "The KM10544 IRAK4 inhibitor has the potential to treat malignancies such as Waldenström's Macroglobulinemia with MYD88 mutation and others. We look forward to furthering the development of this exciting compound and are grateful for our continuing collaboration with the Kainos team."
Kisub Lee, Chairman and Chief Executive Officer of Kainos, added, "We have devoted significant efforts to bring KM10544 to its current stage and we are confident that Emmaus' drug development expertise will allow the company to continue to progress this important program through the clinic."
IRAK4 (interleukin-1 receptor-associated kinase 4) is a serine-threonine protein kinase made up of 460 amino acids that is a key messenger in signaling initiated by TLR/IL-1R (toll-like receptor/interleukin-1 receptor). It is known to be present and active in the occurrence of certain immunity responses, inflammation disorders and various cancers with myeloid differentiation factor 88 (MYD88) mutation.
About Kainos Medicine
Kainos Medicine, Inc., based in South Korea, is a clinical-stage company that specializes in the research and development of innovative medicines for brain diseases, cancers and infectious diseases, led by researchers who have years of experience and success in new drug discovery and development and an executive team that has experience with NASDAQ-listed companies. Kainos receives specialized counsel on product selection, evaluation and external licensing deals from advisory boards and business development consultants who have skills and significant experience in global drug development. For more information, please visit .
About Emmaus Life Sciences
Emmaus Life Sciences, Inc. is a commercial-stage biopharmaceutical company and leader in the treatment of sickle cell disease. The company currently markets U.S. Food and Drug Administration approved ENDARI® (L-glutamine oral powder) indicated to reduce the acute complications of sickle cell disease in adults and children 5 years and older. The company is also engaged in the discovery and development of innovative treatments and therapies for certain rare and orphan diseases as well as those affecting larger populations, such as diverticulosis. For more information, please visit .
Forward-looking Statements
This press release contains forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995, as amended, including statements regarding the potential to advance early-stage studies of KM10544. These forward-looking statements are subject to numerous assumptions, risks and uncertainties which change over time, including uncertainties and risk factors disclosed in the company's 2020 Annual Report on Form 10-K/A filed with the SEC on August 10, 2021, and actual results may differ materially. Such forward-looking statements speak only as of the date they are made, and Emmaus assumes no duty to update them, except as may be required by law.
Company Codes: OTC-PINK:EMMA
合作孤儿药引进/卖出
分析
对领域进行一次全面的分析。
登录
或

生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用

