预约演示
更新于:2026-03-05
Sacituzumab govitecan-hziy
戈沙妥组单抗
更新于:2026-03-05
概要
基本信息
药物类型 ADC |
别名 hRS7-SN38 antibody drug conjugate、Isactuzumab govitecan、Sacituzumab Govitecan + [13] |
作用方式 抑制剂 |
作用机制 TOP1抑制剂(DNA拓扑异构酶I抑制剂)、Trop-2抑制剂(肿瘤相关钙信号传感器2抑制剂) |
在研适应症 |
最高研发阶段批准上市 |
首次获批日期 美国 (2020-04-22), |
最高研发阶段(中国)批准上市 |
特殊审评优先审评 (美国)、突破性疗法 (美国)、加速批准 (美国)、孤儿药 (美国)、优先审评 (中国)、附条件批准 (中国)、孤儿药 (韩国)、优先审评 (澳大利亚)、快速通道 (韩国)、优先审评 (中国台湾)、孤儿药 (澳大利亚)、快速通道 (美国) |
登录后查看时间轴
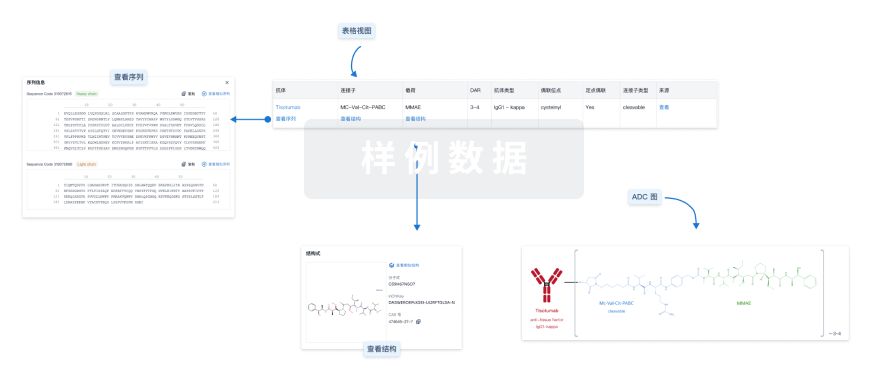
结构/序列
使用我们的ADC技术数据为新药研发加速。
登录
或
Sequence Code 27958L

来源: *****
Sequence Code 319372635H

来源: *****
关联
134
项与 戈沙妥组单抗 相关的临床试验NCT07393555
A Randomized Phase II Study of Sacituzumab Govitecan Alone, Ivonescimab Alone, or Sacituzumab Govitecan and Ivonescimab in Participants With Previously-Treated Actionable Genomic Alteration Positive Stage IV or Recurrent Non-Small Cell Lung Cancer (Lung-MAP Sub-Study)
This phase II Expanded Lung-MAP treatment trial compares how well sacituzumab govitecan alone, ivonescimab alone, or sacituzumab govitecan in combination with ivonescimab works in treating patients with non-small cell lung cancer (NSCLC) that has come back after a period of improvement (recurrent) or is stage IV and has a change in at least 1 of these genes: ALK, EGFR, HER2 (ERBB2), MET, NTRK, RET, and ROS1. This type of gene change is called an actionable genomic alteration (AGA), which means certain treatments can target the change to fight the cancer. Sacituzumab govitecan is a monoclonal antibody, called sacituzumab, linked to a toxic drug called SN-38. Sacituzumab govitecan is a form of targeted therapy because it attaches to specific molecules (receptors) on the surface of tumor cells, known as TROP2 receptors, and delivers SN-38 to kill them. Ivonescimab is a bispecific antibody that can bind to two different antigens at the same time. It binds to programmed cell death protein 1 (PD1), a protein found on the surface of T cells (a type of white blood cell) and vascular endothelial growth factor (VEGF), a protein found on the surface of tumor cells. Ivonescimab may strengthen the immune system and interfere with the ability of tumor cells to grow and spread. Giving a combination of sacituzumab govitecan and ivonescimab work better than either drug alone, and sacituzumab govitecan alone, ivonescimab alone, or sacituzumab govitecan and ivonescimab together may work better than standard treatments at shrinking NSCLC with an AGA.
开始日期2026-08-07 |
申办/合作机构  SWOG SWOG [+1] |
NCT07367178
Phase II Study to Improve Sacituzumab Govitecan Tolerance With Atropine in Patients With Advanced Triple-Negative and Hormone Receptor-Positive/HER2-Negative Breast Cancer
The SATROPIN study is an international, multicenter, open-label, single-arm, phase II clinical trial to assess whether the use of prophylactic administration of atropine may prevent diarrhea in participants with unresectable locally advanced or metastatic triple-negative breast cancer (TNBC) or hormone receptor-positive/HER2-negative (HR(+)/HER2-) treated with sacituzumab govitecan.
开始日期2026-07-01 |
NCT07328490
A Phase I/II Study to Assess the Safety and Antitumor Activity of Bispecific T-cell Engager Tarlatamab and TROP2 Targeted Antibody Drug Conjugate Sacituzumab Govitecan in Previously Treated Extensive-Stage Small Cell Lung Cancer and Extrapulmonary Neuroendocrine Cancer
Background:
Small-cell lung cancer (SCLC) is the most deadly form of lung cancer. It kills at least 250,000 worldwide each year. Extra-pulmonary neuroendocrine cancer (EP-NEC) is a similar type of cancer that develops anywhere other than the lungs. EP-NEC is also very aggressive. Better treatments are needed for these cancers.
Objective:
To test 2 drugs (tarlatamab combined with sacituzumab govitecan [SG]) in people with SCLC or EP-NEC.
Eligibility:
People aged 18 years and older with SCLC or EP-NEC that either did not respond to or returned after treatment.
Design:
Participants will be screened with a physical exam, blood tests, heart function testing, and imaging scans.
Both study drugs are given intravenously (through a needle in the arm). Participants will receive a small starter dose of tarlatamab (1 mg) 2 weeks before beginning regular treatment, followed by the full dose (10 mg) one week later. Treatment then follows a repeating 4-week cycle: tarlatamab (10 mg) on days 1 and 15, and sacituzumab govitecan (7.5 or 10 mg/kg) on days 1 and 8. Treatment continues for up to 2 years, unless the cancer worsens, the participant passes away, or side effects become too severe.
Participants will have regular check-ups including physical exams, blood tests, and imaging scans to monitor safety and treatment response. Blood and tumor samples will be collected for research purposes.
After stopping treatment, participants will return for a safety check at 30 days, then be contacted every 3 months to check on their health and survival. Those who stop treatment for reasons other than cancer progression will continue CT scans every 8 weeks until their disease progresses.
Small-cell lung cancer (SCLC) is the most deadly form of lung cancer. It kills at least 250,000 worldwide each year. Extra-pulmonary neuroendocrine cancer (EP-NEC) is a similar type of cancer that develops anywhere other than the lungs. EP-NEC is also very aggressive. Better treatments are needed for these cancers.
Objective:
To test 2 drugs (tarlatamab combined with sacituzumab govitecan [SG]) in people with SCLC or EP-NEC.
Eligibility:
People aged 18 years and older with SCLC or EP-NEC that either did not respond to or returned after treatment.
Design:
Participants will be screened with a physical exam, blood tests, heart function testing, and imaging scans.
Both study drugs are given intravenously (through a needle in the arm). Participants will receive a small starter dose of tarlatamab (1 mg) 2 weeks before beginning regular treatment, followed by the full dose (10 mg) one week later. Treatment then follows a repeating 4-week cycle: tarlatamab (10 mg) on days 1 and 15, and sacituzumab govitecan (7.5 or 10 mg/kg) on days 1 and 8. Treatment continues for up to 2 years, unless the cancer worsens, the participant passes away, or side effects become too severe.
Participants will have regular check-ups including physical exams, blood tests, and imaging scans to monitor safety and treatment response. Blood and tumor samples will be collected for research purposes.
After stopping treatment, participants will return for a safety check at 30 days, then be contacted every 3 months to check on their health and survival. Those who stop treatment for reasons other than cancer progression will continue CT scans every 8 weeks until their disease progresses.
开始日期2026-03-09 |
申办/合作机构 |
100 项与 戈沙妥组单抗 相关的临床结果
登录后查看更多信息
100 项与 戈沙妥组单抗 相关的转化医学
登录后查看更多信息
100 项与 戈沙妥组单抗 相关的专利(医药)
登录后查看更多信息
1,888
项与 戈沙妥组单抗 相关的文献(医药)2026-04-01·CRITICAL REVIEWS IN ONCOLOGY HEMATOLOGY
Recent advances in systemic treatment for HER2-negative breast cancer with brain metastases
Review
作者: Zhu, Yuehong ; Hao, Chunfang ; Zhang, Jie
Human epidermal growth factor receptor 2 (HER2) -negative breast cancer brain metastases (BCBM) and leptomeningeal disease (LMD) pose significant clinical challenges due to limited treatment options and poor prognosis. Unlike HER2-positive BCBM, which has established therapeutic protocols, HER2-negative BCBM and LMD lacks standardized approaches. Recent advances have introduced novel systemic therapies targeting diverse pathways, including cell cycle regulation, DNA repair, immune modulation, vascular suppression, and leptomeningeal penetration, with emerging data supporting activity in LMD. Cyclin-dependent kinase 4/6 (CDK4/6) inhibitors show promise in hormone receptor-positive subsets, particularly when combined with radiotherapy. Antibody-drug conjugates (ADCs), such as trastuzumab deruxtecan and sacituzumab govitecan, demonstrate enhanced central nervous system penetration and tumor-specific cytotoxicity, improving outcomes in both stable and active brain metastases. Immune checkpoint inhibitors (ICIs) and poly (ADP-ribose) polymerase (PARP) inhibitors also exhibit potential, though their efficacy varies by molecular subtype. Additionally, intrathecal therapies have become a cornerstone for LMD management, bypassing the blood-CSF barrier. Emerging therapies like selective estrogen receptor degraders (SERDs) and kinase inhibitors address resistance mechanisms, offering new avenues for personalized treatment. This review underscores the need for survival-prolonging, quality-of-life-preserving strategies with optimized safety profiles, highlighting the evolving landscape of HER2-negative BCBM and LMD management.
2026-03-01·CANCER LETTERS
ABCG2-mediated SN-38 efflux drives resistance to Sacituzumab govitecan in urothelial carcinoma
Article
作者: Rupp, Luis ; Nössing, Christoph ; Lemberger, Ursula ; Grausenburger, Reinhard ; Prantl, Isabella ; Herek, Paula ; Baumfried, Oliver ; Marko, Doris ; Shariat, Shahrokh F ; Bastos-Moreira, Yuri ; Compérat, Eva ; Laukhtina, Ekaterina ; Borsos, Eszter ; Hauser, Lena Marie ; Pirker, Christine ; Wasinger, Gabriel ; Suleja, Agata ; Berger, Walter ; Oszwald, André ; Englinger, Bernhard ; Ertl, Iris Elisabeth ; Valcanover, Daniel ; Gabler-Pamer, Lisa ; Müller, Julia
2026-02-05·ONCOLOGIST
Effectiveness of sacituzumab govitecan in metastatic triple-negative breast cancer: a real-world retrospective cohort study from Central Europe
Article
作者: Ciszewski, Tomasz ; Żubrowska, Justyna ; Krejčí, Daniel ; Rušinová, Lenka ; Smok-Kalwat, Jolanta ; Bielčiková, Zuzana ; Šustr, Jan ; Szymanik-Resko, Magdalena ; Lisik-Habib, Maja ; Holánek, Miloš ; Študentová, Hana ; Pękala, Anika ; Winsko-Szczęsnowicz, Karolina ; Młodzińska, Agnieszka ; Pieniążek, Małgorzata ; Püsküllüoğlu, Mirosława ; Malejčíková, Miroslava ; Jarząb, Michał ; Łacko, Aleksandra ; Polakiewicz-Gilowska, Anna ; Czartoryska-Arłukowicz, Bogumiła ; Pacholczak-Madej, Renata ; Konieczna, Aleksandra ; Soumarová, Renata ; Kolářová, Iveta ; Danielewicz, Iwona
Abstract:
Background:
Sacituzumab govitecan (SG), an antibody–drug conjugate targeting TROP-2, demonstrated superior efficacy over standard chemotherapy in heavily pretreated metastatic triple-negative breast cancer (mTNBC) in the ASCENT trial. However, real-world data remain limited. This study evaluated the effectiveness and safety of SG in an unselected multinational cohort of patients with mTNBC.
Methods:
This retrospective analysis included 303 women who initiated SG treatment between August 2021 and April 2025 across 18 oncology centers in Poland, the Czech Republic and Slovakia, within the Central European Breast Cancer Collaboration (CEBCC-102). Primary endpoints were median progression-free survival (mPFS) and overall survival (mOS). Secondary objectives included response pattern, safety and identification of factors influencing outcomes. Adverse events (AEs) were graded using Common Terminology Criteria for AE, version 5.0, and treatment response was assessed according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1.
Results:
The median follow-up was 8.8 months (IQR 4.5-14.4). The mPFS was 4.4 months, and mOS was 11.3 months. The overall response rate was 30.7%. The most frequent AEs were hematologic: neutropenia (69.0%) and anemia (39.6%). In multivariate Cox analyses, poor ECOG performance status and liver metastases were independently associated with worse outcomes. Diarrhea and hypersensitivity reactions showed favorable prognostic associations.
Conclusions:
In this largest real-world cohort, the clinical benefit of SG observed in the ASCENT trial was confirmed under routine practice conditions. Poor performance status and liver metastases predicted inferior outcomes, while certain treatment-related AEs warrant further investigation.
1,827
项与 戈沙妥组单抗 相关的新闻(医药)2026-03-04
·肿瘤界
孙月丽, 黄婕, 潘燚, 董嵩, 涂海燕, 杨学宁, 杨衿记, 周清, 吴一龙. 历史转折关头的小细胞肺癌[J]. 循证医学, 2026, 26(1): 1-16. DOI: 10.12019/j.issn.1671-5144.202601045
前 言
小细胞肺癌(small-cell lung cancer,SCLC),作为肺癌中恶性程度最高的类型,目前依旧是临床治疗的难点。然而,随着tarlatamab、芦比替定(lurbinectedin)二线治疗地位的确立、一线维持治疗的探索、多样化抗体偶联药物(antibody-drug conjugate,ADC)的崛起以及分子分型的不断优化,SCLC的治疗格局正在发生前所未有的改变:从“全员化疗”逐步向“基于靶点的免疫/ADC治疗”转变。本文分别从分子分型、局部治疗、全身治疗、肺神经内分泌肿瘤和转化性SCLC五个方面评述近年来SCLC的披荆斩棘之路。
1SCLC分子分型进展
1926年,Barnard首次将SCLC及其支气管起源描述为一种罕见的纵隔燕麦细胞肉瘤[1]。大约30年后,Azzopardi对其进行了光学显微镜下的描述,并将SCLC与其他类型肺癌区分开来[2]。随着分子生物学技术的飞速发展,对SCLC的认识从传统形态学特征逐步深入到分子层面。研究发现绝大多数SCLC的癌变发生需要抑癌基因TP53和RB1同时失活,这些双重失活事件通常发生于大气道表达achaete-scute同源物1(achaete-scute homologue 1,ASCL1)的肺神经内分泌细胞,但也可能发生于上皮细胞,如Ⅱ型肺泡细胞、基底细胞或杯状细胞,从而启动SCLC的发生发展[3, 4]。除了TP53和RB1缺失外,SCLC中还存在其它一些反复出现的遗传改变,如MYC扩增、PIK3A激活突变和PTEN缺失等[5, 6]。
长期以来,SCLC被视为一种“同质性疾病”,所有患者都接受相同的治疗方案。然而随着分子病理、测序分析和临床前模型的发展,越来越多的证据表明SCLC是一类具有高度异质性的疾病,分子分型的探索也应运而生,这为SCLC个性化治疗策略的探索奠定了重要基础。现对SCLC分子分型的历史演变进行系统梳理,总结各阶段的核心成果与科学意义。
1.1
早期形态学分型阶段(1985年):基于神经内分泌特征的初步分类
SCLC的分型研究始于形态学与生物学特征的观察。1985年,Carney等[7]首次提出基于细胞形态及神经内分泌特性的二分法分类体系,将SCLC分为经典型(classic)和变异型(variant)两大亚型,这是SCLC分型的开创性探索。该研究共收集 45 例经组织学确诊 SCLC 患者的 400 份标本,从120 份可见肿瘤细胞的标本中成功建立 50 个独立SCLC 细胞系。根据相差显微镜及4种标志物的表达模式[L-多巴脱羧酶(L-dopa decarboxylase, DDC);蛙皮素样免疫反应性(bombesin-like immunoreactivity, BLI);神经元特异性烯醇化酶(neuron-specific enolase, NSE);肌酸激酶脑同工酶(brain isozyme of creatine kinase, CK-BB)]将 50 个细胞系分为两大亚型:经典型和变异型。经典型(35个细胞系,70%)表现为4 种标志物均显著升高,且电镜下多可见神经分泌颗粒,形态学多为紧密堆积或相对致密的漂浮聚集物。而变异型(15 个细胞系,30%)缺乏 DDC 或 BLI 表达(14 个同时缺乏两者),但仍高表达 NSE 和 CK-BB,电镜下部分无神经分泌颗粒(4/15 个),形态学多为松散粘附的漂浮聚集物或贴壁生长。
这一阶段的分型核心依据是肿瘤的形态学表现和神经内分泌属性,明确了SCLC作为神经内分泌肿瘤的基本生物学特征,为后续研究奠定了病理基础。但该分型仅依赖形态学观察,缺乏分子层面的支撑,难以解释肿瘤异质性及治疗响应差异的内在机制。
1.2
转录因子驱动分型阶段(2013—2019年):分子标志物主导的亚型细分
随着转录组学技术的应用,SCLC分型研究进入分子标志物驱动的新阶段,核心转录因子的发现推动了分型体系的精细化,逐步形成了以关键转录因子表达为核心的分类标准。
1.2.1 双转录因子分型探索(2013年)
2013年,Poirier等[8]通过对SCLC细胞系的转录组分析,发现ASCL1和NEUROD1两种关键转录因子,它们作为正常神经内分泌发育的核心调控因子,在SCLC中呈现特异性高表达且表达模式互不重叠。据此提出二分法分子分型:ASCL1高表达亚型(SCLC-A)和NEUROD1高表达亚型(SCLC-N)。SCLC-A亚型对应传统经典型SCLC,SCLC-N亚型则对应变异型SCLC。这一发现首次揭示了SCLC亚型差异的分子基础,证实转录因子表达模式可作为分型的核心依据。
1.2.2 三分型体系建立(2016年)
2016年,Borromeo等[9]对38例人源化SCLC细胞系进行微阵列分析,在ASCL1和NEUROD1分型基础上,发现部分肿瘤呈现两种转录因子均无/低表达的特征,从而扩展为三分型体系:ASCL1-High、NEUROD1-High及双低表达型。研究证实ASCL1-High亚型依赖ASCL1实现肿瘤形成,调控MYCL1、INSMI、SOX2和DLL3等基因,具备典型神经内分泌表型;NEUROD1-High亚型则依赖MYC扩增驱动,形态学上为变异型特征;双低表达型肿瘤的分子机制尚未明确,但已显示出与前两型不同的转录组特征,为后续新亚型的发现埋下伏笔。
1.2.3 新转录因子发现与四分型确立(2017—2019年)
2017年,McColl等[10]通过对51个SCLC细胞系和肿瘤样本的基因表达谱分析,发现神经内分泌转录因子INSM1与Hippo通路关键介质YAP1 呈 “相互排斥” 的表达模式,INSM1高表达、YAP1低表达亚型(Group Ⅰ,占绝大多数)符合经典SCLC的神经内分泌表型,对化疗药物(伊立替康、顺铂、依托泊苷)敏感。而INSM1低表达、YAP1高表达亚型(Group Ⅱ,占少数)对化疗耐药,预后差,后被命名为SCLC-Y亚型。
2018年,Huang等[11]进一步发现转录因子POU2F3在另一部分双低表达型肿瘤中特异性高表达,该亚型约占SCLC的12%,仅见于神经内分泌标志物低表达的肿瘤,且上调Tuft细胞标志物,提示其起源于Tuft细胞,命名为SCLC-P亚型。
2019年,Rudin等[12]整合29例组织样本、52例组织样本的基因组测序数据及54株SCLC细胞系的RNA测序(RNA sequencing,RNA-seq)数据,通过层次聚类分析,正式确立了SCLC的四分型标准:SCLC-A(ASCL1高表达)、SCLC-N(NEUROD1高表达)、SCLC-P(POU2F3高表达)和SCLC-Y(YAP1高表达)。该分型体系明确了各亚型的核心分子特征:SCLC-A占比最高(约70%),δ样配体3(delta-like ligand 3,DLL3)和B细胞淋巴瘤-2蛋白(B-cell lymphoma-2,BCL-2)表达较高;SCLC-N占比11%,MYC扩增常见;SCLC-P占比16%,无神经内分泌特性;SCLC-Y占比仅2%,Hippo通路激活,预后较差且易化疗耐药。这一分型成为SCLC分子分型的经典框架,为后续临床转化研究提供了统一标准。
1.3
多维度整合分型阶段(2021—2025年):免疫特征与多组学的融合
近年来,随着免疫治疗的兴起及多组学技术的发展,SCLC分型研究进一步整合免疫微环境特征、多组学数据及临床治疗响应,形成了更具临床指导意义的整合型分型体系。
1.3.1 炎症亚型的提出与四分型优化(2021年)
2021年,Gay等[13]利用1 300个基因的表达数据进行分层聚类,在经典四分型基础上,发现部分双低表达型肿瘤伴有显著的炎症基因特征,将其定义为SCLC-I(炎症性)亚型,形成新的四分型:SCLC-A(神经内分泌型)、SCLC-N(神经内分泌型)、SCLC-P(非神经内分泌型)和SCLC-I(非神经内分泌型)。该研究首次将免疫特征纳入分型标准,证实SCLC-I亚型肿瘤(免疫相互作用增强),可能对Bruton’s酪氨酸激酶(Bruton’s tyrosine kinase,BTK)抑制剂敏感,且从免疫联合化疗中获益最大;SCLC-A亚型对BCL-2抑制剂敏感;SCLC-N亚型对极光激酶(aurora kinase,AURK)抑制剂敏感;SCLC-P亚型对聚腺苷二磷酸核糖聚合酶(poly (ADP-ribose) polymerase,PARP)抑制剂敏感。同时发现,铂类化疗耐药可诱导亚型转换,如SCLC-A在顺铂治疗后可向SCLC-I转变,揭示了SCLC亚型的动态可塑性。
1.3.2 多组学驱动的新分型探索(2024—2025年)
2024年,中国学者Liu等[14]通过前瞻性收集112例未经治疗的原发性SCLC肿瘤样本,整合全外显子测序(whole exome sequencing,WES)、RNA-seq、蛋白组及磷酸化蛋白组学数据,采用无监督聚类分析提出NMF1-4四分型:NMF1亚型高表达ASCL1和NEUROD1,E2F活性高,对化疗敏感;NMF2亚型高表达ASCL1和DLL3,适合靶向DLL3治疗;NMF3亚型高表达炎性因子,RTK信号通路激活,对靶向RTK治疗敏感;NMF4亚型高表达POU2F3,MYC信号通路激活,对AURK抑制剂敏感。该分型首次结合多组学数据,实现了分子特征与治疗靶点的精准匹配。同年,Nabet等[15]对IMpower133研究的271例肿瘤组织进行RNA-seq分析,结合神经内分泌表型和免疫浸润状态,将SCLC-I亚型进一步细分为NE型(SCLC-I-NE)和非NE型(SCLC-I-nNE),形成NMF1-NMF4优化分型:NMF1(NEUROD1驱动,免疫冷)、NMF2(ASCL1驱动,免疫冷)、NMF3(ASCL1驱动,免疫热)、NMF4(POU2F3驱动,免疫热)。研究证实,免疫热亚型(NMF3、NMF4)的免疫治疗效果显著优于免疫冷亚型,且NE肿瘤细胞比例与预后密切相关,低肿瘤相关巨噬细胞(tumor-associated macrophage,TAM)、高效应T信号的NE肿瘤患者生存期更长。此外,Park等[16]通过整合427例SCLC样本的免疫组化、靶外显子组测序和RNA-seq数据,提出基于免疫组化的(immunohistochemistry-based,IHC-based)五分型(A、AN、N、P、TN型),并证实炎症性肿瘤(占25%)对一线免疫治疗的响应显著优于非炎症性肿瘤,进一步验证了免疫特征在分型中的临床价值。Huo等[17]则基于蛋白质组学聚类,提出S-Ⅰ、S-Ⅱ、S-Ⅲ三分型,其中S-Ⅱ亚型富集干扰素及炎症反应通路,免疫治疗应答最佳,S-Ⅲ亚型预后最差,为化疗耐药患者提供了新的治疗靶点探索方向。
2025年,Chen等[18]纳入165例SCLC患者,采用多重免疫荧光染色(co-detection by indexing,CODEX)多重成像、WES、RNA-seq等技术,生成包含930余万个细胞的267张高维图像,结合长期随访数据(中位随访62.3个月)开展分析。识别出多阳性肿瘤细胞(multi-positive tumor cells,MPTC),即共表达两种及以上转录因子的肿瘤细胞,占肿瘤细胞的10%以上。在SCLC-A亚型中,MPTC富集的细胞邻域(cellular neighborhood,CN9)具有高增殖、高转移潜能,高表达SLFN11基因,与不良预后相关。SCLC整体免疫浸润水平较低,呈现“冷肿瘤”特征,但SCLC-Y亚型免疫浸润最高。开发ColonyMap算法,识别出由抗肿瘤巨噬细胞、CD8+ T细胞和自然杀伤T细胞组成的空间聚集免疫生态位(microenvironmental tumor types,MT2)。MT2生态位与患者良好生存显著相关,且不依赖整体免疫浸润水平。以M1样巨噬细胞为核心的MT2生态位,可预测SCLC患者对程序性细胞死亡配体1(programmed death ligand-1,PD-L1)抑制剂免疫治疗的应答,高比例M1-MT2生态位患者的治疗生存期更长。但该研究聚焦于局限期、未接受过系统治疗的SCLC患者,结果能否推广至转移性或经治患者尚不明确,基于组织芯片的分析可能受肿瘤内异质性影响,而且潜在生物标志物的临床价值需前瞻性试验进一步验证。
基于SCLC分子分型的不断发展,SCLC现有诊断和分期是否需要进一步优化、是否根据分子分型制定相应治疗策略均是未来亟需回答的问题。
2SCLC局部治疗进展
几十年来,手术切除一直被排除在SCLC的治疗手段之外,这主要是因为20世纪70年代和90年代基于手术的随机临床试验结果不尽如人意[19, 20]。尽管如此,对于T1-2N0M0患者,肺叶切除术联合纵隔淋巴结清扫术后辅助化疗的3年总生存(overall survival,OS)率超过50%[21, 22]。此外,最近的倾向性评分匹配试验报道,即使是疾病分期更晚的手术治疗患者,也获得了令人鼓舞的长期生存率[23, 24]。然而,需注意的是,只有在应用多周期辅助全身化疗的情况下,手术切除才能获得持久的长期效果[22, 25]。在一项基于大型人群队列的研究中,与未接受辅助治疗相比,接受辅助化疗联合或不联合放疗的患者5年OS率显著提高了12%以上。这甚至高于非小细胞肺癌(non-small cell lung cancer,NSCLC)术后化疗报道的绝对获益(基于顺铂的化疗使5年OS率提高了5.4%)[25, 26]。因此,当前的临床实践指南建议所有T1-2N0M0的SCLC接受肺切除术的患者都应接受辅助化疗。获得持久长期结局的其他重要预测因素包括解剖性肺切除术(首选肺叶切除术)、性别(女性具有显著的生存优势)以及病理证实的肿瘤完全切除(R0切除)[27-29]。在未来,循环肿瘤细胞或微小残留病灶(minimal residual disease,MRD)的术后检测结果为阴性,可能会进一步完善R0切除的准确定义[30]。
近年来随着免疫治疗在肺癌中的广泛应用,越来越多研究探索在局限期SCLC进行免疫化疗诱导后评估手术的治疗模式 [31, 32]。Zhu等[33]的回顾性分析报道了Ⅰ~ⅢB期接受诱导化疗或化疗联合免疫治疗的效果,其中29例接受免疫化疗诱导治疗,24例接受了化疗。免疫化疗治疗组客观缓解率(objective response rate,ORR)为89.5%,有14例接受了手术(48.3%,14/29),主要病理缓解(major pathological response,MPR)率为57.1%,病理完全缓解(pathological complete response,pCR)率为50.0%。免疫化疗治疗组ORR为75%,有5例接受手术(20.8%,5/24),MPR率为40.0%,pCR率为0.0%。但两组的中位无病生存期(disease-free survival,DFS)均未达到,故暂无法评价诱导免疫对生存的影响。Zhao等[34]的多中心回顾性数据分析了139例局限期SCLC治疗的效果,其中55例接受了同期化放疗,56例新辅助化疗后手术,28例在新辅助免疫化疗后进行了手术,所有手术患者都达到R0切除。免疫化疗组的pCR率(35.7% vs. 7.1%,P < 0.05)和MPR率(53.6% vs. 12.5%,P < 0.05)均显著高于化疗组,经过倾向性评分匹配后,各组有24例患者进行了生存分析,免疫化疗组的DFS和OS均未达到,但Kaplan-Meier生存分析显示新辅助免疫化疗组的DFS和OS显著长于新辅助化疗组或同期化放疗组。Sun等[35]发表的单臂Ⅱ期临床研究(LungMate-005)报道了给予ⅠB~ⅢC期的患者接受抗PD-L1抗体TQB2450诱导治疗后多学科会诊(multidisciplinary team,MDT)评估局部治疗方案,95%的患者为Ⅲ期,诱导治疗后ORR达到92.5%。27例患者评估可手术,其中21例接受手术治疗,手术的患者MRP率为61.9%,pCR率为42.9%。中位无事件生存期(event-free survival,EFS)为16.2个月,24个月EFS率为66.7%,24个月OS率为85.7%。14例进行放疗治疗的患者中,有6例拒绝手术,6例仍评估不可手术,2例因计划的手术切除范围过大而选择放疗。放疗组24个月EFS率为14.3%,24个月OS率为42.9%。Huang等[36]的真实世界研究中,纳入的25例患者在接受新辅助免疫化疗后的ORR达84%。经过MDT评估后,有16例接受手术,9例接受巩固放疗。接受手术的患者分析显示pCR率为50%,MPR率为56.3%,18个月EFS率和OS率分别为76.6%和80.8%。值得注意的是,pCR患者均未出现肿瘤进展。手术治疗组18个月EFS率显著优于免疫化疗+放疗组(33.3%)[风险比(hazard ratio,HR)=4.066, P=0.032],但18个月OS率(100%)未达统计学显著性。上述研究表明新辅助免疫化疗相对于新辅助化疗,可显著提高手术切除后的pCR率和MRP率,也展现出延长患者的EFS和OS的潜力,但目前研究样本量较小,随访时间也不足。
自1992年荟萃分析[37, 38]明确胸部放疗可改善局限期SCLC预后以来,以化疗为基础的早期同步放化疗(concurrent chemoradiotherapy,CCRT)逐渐确立为标准治疗。然而,此后20多年总体疗效一直处于瓶颈期。直到2024年ADRIATIC研究[39]公布CCRT后度伐利尤单抗巩固治疗相比安慰剂,中位OS提高了22.5个月(55.9个月 vs. 33.4个月;HR=0.73,P=0.010 4),中位无进展生存期(progression-free survival,PFS)延长了7.7个月(16.6个月 vs. 9.2个月;HR=0.76,P=0.016 1),且整体耐受性良好。该研究凭借亮眼的生存数据推动CCRT后巩固免疫成为新的标准治疗。王子与公主的童话故事似乎完美落幕了,然而在大家相信放疗序贯免疫治疗产生协同效应时,2025年美国临床肿瘤学会(American Society of Clinical Oncology,ASCO)报道的CCRT后巩固免疫治疗的Ⅱ期ACHILES研究,当主角换成阿替利珠单抗后,对比安慰剂却是阴性结果,生存并未改善[40]。相同的设计不同的结果,使放疗序贯免疫治疗的协同效应仍存在不确定性。
在巩固免疫获得突破后,免疫治疗进一步前移至CCRT是否能增加获益呢?随机对照Ⅲ期研究NRG LU005[41]探索CCRT同步阿替利珠单抗后巩固阿替利珠单抗1年在局限期SCLC的疗效,结果显示对比安慰剂无论OS、PFS或DFS均无差异,甚至中位OS略短,结合NSCLC的PACIFIC2[42]、CheckMate 73L[43]、KEYNOTE-867[44]研究,至此肺癌CCRT同步免疫的研究均以失败告终。究其原因可能是放疗的免疫抑制、同步毒性叠加,特别是放射性/免疫性肺炎不良反应发生率增加,削弱了免疫治疗的获益。
如何进一步提升局部控制,在免疫治疗时代优化放疗方式呢?ADRIATIC研究中度伐利尤单抗巩固治疗仅降低了远处转移风险,并未减少胸腔内复发的发生,肺及纵隔复发率仍然超过1/4[39]。基于“增加放疗剂量能降低局部复发进而提高生存”的假说,国内开展一项Ⅲ期研究对比54 Gy/30 F(1.8 Gy/ F,bid)与45 Gy/30 F(1.5 Gy/ F,bid),结果显示提高放疗剂量中位OS达到60.7个月,对照组为39.5个月(HR=0.55,P=0.003),中位PFS 为 30.5个月vs. 16.7 个月(HR=0.70,P=0.044),两组毒性反应无显著差异[45],提示在安全性可控的情况下提高放疗剂量可改善局部控制并带来生存获益。另一项Ⅱ期研究将剂量提升至60 Gy(1.5 Gy/ F,bid)同样显示中位PFS及OS延长[46],进一步支持剂量优化的研究方向。
因此,在免疫治疗的背景下,新辅助免疫联合治疗后手术的模式能否真正取代现有的标准CCRT后免疫巩固治疗模式,仍需前瞻性的随机对照研究进一步验证。
此外,在广泛期SCLC(extensive-stage SCLC,ES-SCLC)的治疗版图中,放疗也占有一席之地。在化疗时代,对于一线化疗有效的ES-SCLC患者进行胸部巩固放疗可使2年OS率提高10%。但由于一线标准治疗变迁为化疗免疫后,胸部放疗的增益尚缺乏强有力的循证医学证据。针对这个问题,山东省肿瘤医院开展Ⅱ期单臂研究,对一线化疗免疫有效的患者进行胸部巩固放疗联合免疫维持治疗,中位OS达到21.4个月,中位PFS达到10.1个月,安全性可控[47],但该策略仍需随机对照研究进一步验证。与NSCLC不同,对于已有脑转移的SCLC,传统治疗是全脑放疗(whole brain radiation therapy,WBRT)。然而,随着免疫治疗带来患者生存的延长,WBRT引起的神经认知功能损害日益受到关注。Aizer等开展前瞻性多中心Ⅱ期研究评估1~10个脑转移SCLC进行立体定向放疗,结果显示立体定向放疗可使78%患者避免WBRT,且神经性死亡率(指影像学明显进展伴神经系统症状,且无系统性进展或危及生命的全身症状)显著低于历史WBRT对照组[48],提示在特定人群中立体定向放疗有望成为兼顾疗效与生活质量的重要选择。
随着免疫检查点抑制剂(immune checkpoint inhibitors,ICIs)、T细胞衔接器、ADC等新药持续推动ES-SCLC生存延长,如何通过细分人群、优化放疗剂量与靶区策略、优化与全身治疗结合方式等,使局部放疗在延长患者生存的同时尽可能降低不良反应、提高患者生活质量,将成为下一阶段的研究主题。
3SCLC全身治疗进展
3.1
一线治疗创新
在免疫治疗问世前,ES-SCLC的一线标准治疗为依托泊苷联合顺铂/卡铂(etoposide + cisplatin / carboplatin,EP/EC)的双药化疗方案。该方案虽能实现50%~70%的ORR,但肿瘤缓解持续时间短,中位OS仅为10~12个月,5年OS率不足5%,且患者极易出现耐药复发[49-51]。由于SCLC具有高增殖、高异质性的生物学特性,单一化疗药物的治疗潜力已达极限,亟需探索新的治疗靶点和联合策略。
免疫治疗的崛起为ES-SCLC的治疗带来了突破性变革,尤其是免疫联合化疗方案的应用显著改善了患者的生存结局。2019年成为ES-SCLC治疗的重要转折点,两项里程碑式的Ⅲ期临床试验IMpower133研究[52]和CASPIAN研究[53]的结果相继公布,确立了免疫联合化疗在ES-SCLC一线治疗中的标准地位。IMpower133研究[52]显示,阿替利珠单抗联合EC方案一线治疗ES-SCLC,相较于单纯化疗,显著延长患者中位OS(12.3个月 vs. 10.3个月)和中位PFS(5.2个月 vs. 4.3个月)。随后的CASPIAN研究[53]证实,度伐利尤单抗联合铂类+依托泊苷同样显著提升ES-SCLC患者的中位OS(13.0个月 vs. 10.3个月),且无论PD-L1表达水平如何,患者均能获益。由此开启了ES-SCLC一线免疫联合治疗的新时代。
尽管化疗联合PD-L1抑制剂方案显著提升了ES-SCLC的一线治疗疗效,但患者的长期生存仍不理想,多数患者在治疗后短期内出现疾病进展。因此,在一线化疗联合免疫治疗达到疾病控制后,探索有效的维持治疗策略,以进一步延缓疾病进展、延长生存时间,成为近年来ES-SCLC治疗研究的重点方向。此前多项关于免疫单药或双免疫联合维持治疗的研究[54, 55](如CheckMate 451、KEYNOTE-604等)均未取得理想的阳性结果,提示单一免疫维持治疗可能难以满足临床需求。2025年公布的Ⅲ期IMforte研究[56]填补了ES-SCLC一线维持治疗的空白,成为首个在一线维持治疗场景中同时达到PFS和OS主要终点的Ⅲ期研究。而近期公布的DeLLphi-303研究[57],首次探索了新型双特异性T细胞衔接器(bispecific T-cell engager,BiTE)药物tarlatamab联合PD-L1抑制剂作为ES-SCLC一线化疗联合免疫治疗后的维持治疗方案,为该领域带来了新的突破,进一步推动了ES-SCLC一线治疗格局的演进。
IMforte研究[56]是一项全球多中心、随机、开放标签的 Ⅲ 期临床试验,旨在评估芦比替定联合阿替利珠单抗对比阿替利珠单抗单药作为ES-SCLC一线维持治疗的疗效与安全性。该研究在2025年ASCO年会上首次公布关键结果,并发表于Lancet杂志。结果显示,芦比替定联合阿替利珠单抗组的中位PFS显著延长(5.4个月 vs. 2.1个月),疾病进展或死亡风险降低46%;中位OS同样显著改善(13.2个月 vs. 10.6个月),死亡风险降低27%。亚组分析显示,除乳酸脱氢酶(lactate dehydrogenase,LDH)高于正常上限、接受过预防性全脑照射(prophylactic cranial irradiation,PCI)的患者外,其余各亚组(包括不同年龄、性别、种族、地区及肝转移状态)均能从联合维持治疗中获益,证实了该方案的广泛适用性。安全性方面,联合治疗组的不良事件(adverse event,AE)发生率高于单药组(97% vs. 81%),但多数AE为1~2级,3~4级AE主要为血液学毒性(贫血、中性粒细胞减少、血小板减少等),且通过预防性使用粒细胞集落刺激因子(granulocyte colony-stimulating factor,G-CSF)可有效管理。联合组因AE导致的治疗中断率仅为6.2%,与单药组(3.3%)接近,表明该联合方案的安全性可控,未出现新的安全信号。
DeLLphi-303研究[57]是一项Ⅰb期、多中心、开放标签、多队列临床研究,核心目的是评估全球首款靶向DLL3的BiTE药物tarlatamab联合标准化疗+免疫治疗,后续序贯tarlatamab联合PD-L1抑制剂维持治疗在ES-SCLC患者中的安全性、药代动力学特征及初步抗肿瘤活性,其维持治疗队列结果于2025年9月发表于Lancet Oncology。该队列共纳入88例经4~6个周期EP方案联合PD-L1抑制剂治疗后未出现疾病进展的ES-SCLC患者,随机分为tarlatamab联合阿替利珠单抗组(48例)和tarlatamab联合度伐利尤单抗组(40例),维持治疗直至疾病进展。研究结果显示,该联合维持治疗方案展现出良好的安全性和优异的疗效。在安全性方面,该联合方案的耐受性良好,未出现剂量限制性毒性或治疗相关死亡事件。最常见的治疗相关不良事件为细胞因子释放综合征(cytokine release syndrome,CRS),发生率为56%,但绝大多数为1~2级(54%),仅1例患者出现3级CRS,且所有CRS事件均通过支持治疗或托珠单抗治疗后完全缓解,中位缓解时间为3天。免疫效应细胞相关神经毒性综合征(immune effector cell-associated neurotoxicity syndrome,ICANS)的发生率为6%,均为1~2级,经支持治疗后可快速缓解,未导致治疗中断或剂量调整。在疗效方面,全人群的中位PFS达到5.6个月,6个月PFS率为47%,12个月PFS率为34%;中位OS高达25.3个月,12个月OS率为82%,18个月OS率为75%,显著优于此前标准治疗方案的生存数据。例如IMpower133研究[52]中阿替利珠单抗联合化疗后阿替利珠单抗维持治疗的中位OS仅为12.3个月,IMforte研究[56]中芦比替定联合阿替利珠单抗维持治疗的中位OS为13.2个月。此外,该联合方案还展现出显著的肿瘤缓解效果,88例患者中有21例(24%)在维持治疗阶段获得了进一步的客观缓解,包括19例部分缓解(partial response,PR)和2例完全缓解(complete response,CR),疾病控制率(disease control rate,DCR)达到60%,其中36%的患者实现了至少52周的持续疾病控制。
DeLLphi-303研究诱导治疗队列(队列2、4、7)的研究结果于2025年欧洲内科肿瘤学会(European Society for Medical Oncology, ESMO)大会公布[58]。该队列聚焦tarlatamab联合标准化疗+免疫治疗,后续衔接tarlatamab联合PD-L1抑制剂(阿替利珠单抗或度伐利尤单抗)维持治疗。研究主要终点为安全性评估(剂量限制性毒性、治疗相关不良事件等),次要终点包括ORR、PFS、缓解持续时间(duration of response,DoR)、DCR及OS等。队列共纳入96例ES-SCLC患者,56例接受tarlatamab+ 依托泊苷+铂类+阿替利珠单抗,40例接受tarlatamab+依托泊苷+铂类+度伐利尤单抗。所有患者均出现治疗相关不良事件(treatment-related adverse event, TRAE),3%的患者出现了剂量限制毒性,3级和4级TRAE发生率分别为43%和35%,与既往已知毒性谱一致,未观察到新的安全信号。ORR达71%,其中CR率为5%,PR率为66%;DCR为82%,DoR为11.0个月,中位疾病控制持续时间10.7个月;39%的患者疾病控制持续至少52周,至数据截止时,49%的患者应答仍在持续,提示疗效具有良好的持久性。中位PFS为10.3个月,12个月PFS率达43.1%,较传统方案(中位PFS通常6~8个月)显著延长;中位OS尚未达到,1年OS率达80.6%,展现出潜在的长期生存获益,进一步印证tarlatamab联合方案一线应用的巨大潜力。
3.2
二线治疗新标杆
尽管一线化疗+PD-L1/PD-1抑制剂治疗可实现初步缓解,但多数患者在1年内出现疾病进展,中位OS仅约12个月。对于铂类治疗失败的患者,二线治疗长期面临疗效有限、毒性显著的困境。过去30年,拓扑替康是标准二线化疗药物[59],但其ORR仅24.3%左右,中位OS为25.9周,且伴随严重血液学毒性;氨柔比星[60]在部分国家获批,但Ⅲ期试验未显示显著生存优势,亟需更有效的治疗方案。
ES-SCLC二线治疗在近5年取得突破性进展,tarlatamab的Ⅲ期数据[61]确立了其在ES-SCLC二线治疗的优选地位,尤其适用于铂类耐药或既往免疫治疗失败的患者;芦比替定可作为铂类敏感患者的重要选择[62]。两者均突破了传统化疗的疗效瓶颈,推动二线治疗从“姑息缓解”向“生存延长”转变。未来需通过更大样本量研究、生物标志物探索和联合治疗策略,进一步优化治疗效果,最终改善患者生存与生活质量。
3.2.1 Tarlatamab:双特异性T细胞衔接器
Tarlatamab是针对DLL3和CD3的BiTE,通过同时结合肿瘤细胞表面的DLL3(85%~96%的ES-SCLC表达)与T细胞表面CD3,激活T细胞杀伤肿瘤,且不依赖MHC-Ⅰ分子识别。2024年5月,美国食品药品监督管理局(Food and Drug Administration,FDA)基于Ⅱ期DeLLphi-301试验[63]结果(10mg推荐剂量组ORR=40%, 9个月OS率估计值为 68%,中位OS为 14.3个月)加速批准tarlatamab用于含铂化疗后进展的ES-SCLC患者,使其成为全球首款获批用于实体瘤的BiTE疗法。2025年ASCO公布Ⅲ期DeLLphi-304试验[61](n=509)的中期分析数据,证实其生存获益优势。Tarlatamab组中位PFS为4.2个月 [95% 置信区间(confidence interval,CI)3.4 ~ 4.5] vs. 3.7个月(95%CI 2.9 ~ 4.2),中位OS达13.6个月(95%CI 11.1~未达到),化疗组8.3个月(95%CI 7.0 ~ 10.2),死亡风险降低40%(HR=0.60, 95%CI 0.47~0.77,P < 0.001),进一步巩固其在SCLC二线治疗中的标准地位。安全性方面可控,相较于化疗,tarlatamab组3级以上AE发生率更低(54% vs. 80%), AE导致停药的发生率也更低(5% vs. 12%)。中国tarlatamab 上市申请于2025年7月获国家药品监督管理局(National Medical Products Administration,NMPA)受理,推进其在全球范围的可及性。
3.2.2 芦比替定:靶向转录调控药物
芦比替定是PharmaMar开发的海鞘素衍生物,结合DNA小沟抑制RNA聚合酶Ⅱ转录抑制肿瘤转录并诱导凋亡,同时调节肿瘤微环境。早期Ⅰ期试验[64]在晚期实体瘤中探索剂量,确立7.0 mg为Ⅱ期推荐剂量(recommended phase 2 dose,RP2D)。Ⅱ期单臂试验[62]使用了3.2 mg/m2的剂量(n=105),研究结果显示,整体ORR为35.2%(95%CI 26.2~45.2),DCR为68.6%;中位OS为9.3个月,中位PFS为3.5个月。铂类敏感患者(化疗无进展间隔≥90天)获益更显著,ORR为45.0%,中位OS为11.9个月;铂类耐药患者(化疗无进展间隔<90天)ORR为22.2%,中位 OS为5.0个月,优于传统化疗。其主要毒性为可逆性骨髓抑制,3~4级中性粒细胞减少发生率为46%,但发热性中性粒细胞减少仅5%;无治疗相关死亡,治疗中断率仅2%。该数据支持FDA加速批准,成为SCLC二线治疗的关键循证依据。2020年6月FDA加速批准用于铂类后进展的转移性SCLC患者。但其确证性Ⅲ期试验ATLANTIS研究[65]使用芦比替定联合治疗对比标准二线治疗(拓扑替康或环磷酰胺+阿霉素+长春新碱),主要终点OS无显著差异[8.6个月vs. 7.6个月,HR=0.97(95%CI 0.82~1.15),P=0.7]。但该研究使用了较低的芦比替定剂量(2.0 mg/m2)且为联合方案,与已获批的单药方案(3.2 mg/m2)存在差异,FDA并未撤销其上市的资格,目前正在开展的LAGOON研究将被视为验证性研究,其结果将决定该药物是否能获得正式获批。中国Ⅰ期桥接临床试验[66]显示,芦比替定3.2 mg/m2 用于ES-SCLC二线治疗,ORR达45.5%,中位PFS为5.6个月,中位OS为11.0个月,安全性与全球数据一致,主要副作用为血液学毒性,提示中国人群获益显著。基于该桥接研究的结果,NMPA于 2024 年底批准芦比替定正式在中国上市,用于治疗既往接受含铂化疗后进展的转移性SCLC患者。
3.3
新型治疗药物进展
3.3.1 新型T细胞衔接器3.3.1.1 ZG006(DLL3三特异性抗体)
ZG006(alveltamig)是全球首个靶向DLL3×DLL3×CD3的三特异性T细胞衔接器(trispecific T-cell activating construct,TriTAC)。ZG006-001是开放、多中心,剂量递增及扩展Ⅰ/Ⅱ期研究,入组经治晚期SCLC/神经内分泌癌,主要终点为ORR和安全性。10 mg Q2W、30 mg Q2W和60 mg Q2W分别纳入5例、16例和12例受试者。有效性方面,截至2025年7月,在SCLC中,10 mg Q2W(4例)、30 mg Q2W组(15例)、60 mg Q2W组(12例)经独立评审委员会(Independent Review Committee,IRC)评估的确认ORR分别为75.0%、60.0%和91.7%,DCR分别为75.0%、73.3%和91.7%。安全性方面,三剂量组的TRAE率均为100%,常见的TRAE有CRS、贫血、白细胞计数减低等,总体安全性良好[67]。2025年启动的Ⅲ期关键试验旨在对比ZG006与标准化疗(拓扑替康/芦比替定)用于一线含铂+免疫失败的SCLC,主要终点为ORR和OS,期待后期数据的公布。
3.3.1.2 Obrixtamig(DLL3×CD3双抗)
Obrixtamig是新型DLL3/CD3 IgG样T细胞衔接器,桥接T细胞杀伤 DLL3阳性肿瘤,低CD3亲和力降低CRS风险。在SCLC的单药[68]后线ORR约21%,安全性可控。Ⅰ期DAREON-8研究探索一线obrixtamig+依托泊苷+卡铂+阿替利珠单抗的疗效,2025年ESMO大会公布其初步数据,可评估患者ORR达68%,DCR达89%,中位DoR为7.3个月,无≥3级CRS或免疫相关AE,安全性与可行性良好,疗效待Ⅱ期确证。
3.3.2 ADC药物3.3.2.1 ZL-1310(DLL3-ADC)
ZL-1310(zocilurtatug pelitecan)是靶向DLL3的ADC,由抗DLL3单抗、肿瘤特异性释放连接子与细胞毒载荷组成。2025年在美国举行的AACR-NCI-EORTC会议上,更新了最新临床数据。其Ⅰ期全球多中心、开放标签、剂量递增(0.8~2.8 mg/kg)研究,共入组 115 例 ES-SCLC,90%经免疫治疗,32%伴脑转移。在102例可评估患者中,全剂量组ORR 为47%(含3例CR)[69]。其中19例接受二线治疗的1.6 mg/kg剂量组ORR最高,经确认的PR率为58%(另有10%未确认)。32例无症状脑转移患者ORR为66%(1例CR,19例PR),在基线时未接受治疗的脑转移患者中也观察到了缓解(8/10)。4例DLL3表达阴性的患者也对治疗产生了反应,ORR为50%。10例曾接受过针对DLL3靶向治疗的患者ORR为40%,其中1例CR,3例PR。安全性方面,总体耐受性良好,115例患者中有23例(20%)报告了≥3 级TRAE。最常见的TRAE是贫血和中性粒细胞减少症。在1.2 mg/kg和1.6 mg/kg剂量下,5.6%的患者因AE减量,无患者因AE停药。Ⅲ期ZL-1310-003于2025年启动,期待ZL-1310在DLL3-ADC药物方面取得突破性进展。
3.3.2.2 Ifinatamab deruxtecan(B7H3-ADC)
Ifinatamab deruxtecan是靶向B7-H3的ADC,由抗B7-H3单抗、可裂解四肽连接子与拓扑异构酶Ⅰ抑制剂DXd偶联,在ES-SCLC后线治疗中显示出较高缓解率与可控安全性。IDeate-PanTumour01是开放标签、多中心Ⅱ期剂量探索研究,入组经治ES-SCLC患者,探索8 mg/kg与12 mg/kg剂量安全性与疗效[70]。Ⅱ期单臂关键试验的IDeate-Lung01研究是国际多中心、开放标签、单臂临床试验,2025年世界肺癌大会(World Conference on Lung Cancer,WCLC)上公布了其主要研究结果。最终选用12 mg/kg剂量组,入组137例含铂化疗后进展的ES-SCLC患者,全人群ORR为48.2%(95%CI 39.6%~56.9%),DCR达87.6%(95%CI 80.9%~92.6%),中位DoR为5.3个月(95%CI 4.0 ~ 6.5),中位PFS为4.9个月(95%CI 4.2 ~ 5.5),9个月OS率为59.1%。安全性方面,任何级别TRAE发生率为89.8%,≥3级TRAE发生率为36.5%,最常见的TRAE为恶心(43.1%)、贫血(34.3%)及中性粒细胞减少症(34.3%)。TRAE相关的停药率为9.5%、死亡率为4.4%。治疗相关的间质性肺疾病(interstitial lung disease,ILD)发生率为12.4%,≥3级ILD发生率为4.4%[71]。目前Ⅲ期随机对照试验IDeate-Lung02研究正在推进,这是一项全球多中心、随机、开放标签临床试验,对比I-DXd(12 mg/kg)与研究者选择化疗(氨柔比星、芦比替定、拓扑替康)用于一线含铂化疗进展的复发SCLC,主要终点为ORR和OS,计划入组约500例。
3.3.2.3 戈沙妥珠单抗(TROP2-ADC)
戈沙妥珠单抗是一款靶向Trop-2的ADC,由抗Trop-2单抗、可裂解连接子与SN-38(拓扑异构酶Ⅰ抑制剂)组成。Trop-2在约71%的SCLC中高表达,与肿瘤侵袭、转移正相关,是理想治疗靶点。Ⅱ期TROPiCS-03研究[72]针对ES-SCLC治疗进展的二线治疗人群。共纳入43例一线含铂+免疫治疗进展的SCLC患者;给药方案为10 mg/kg,D1、D8给药,每21天为一周期。结果显示,全人群ORR为41.9%(95%CI 27.0%~57.9%),均为PR;中位DoR为4.73个月,中位PFS为4.4个月,中位OS为13.6个月。铂敏感(复发间隔≥90天)ORR为47.8%、OS为14.7个月;铂耐药(<90天)ORR为35.0%。安全性方面,3级及以上治疗期间出现的不良事件(treatment emergent adverse events,TEAE)发生率达74.4%,常见为中性粒细胞减少(44.2%)和腹泻(9.3%)。Ⅲ期EVOKE-SCLC-04研究正在进行中,旨在对比戈沙妥珠单抗与拓扑替康/氨柔比星用于ES-SCLC二线治疗,评估有效性与安全性,计划入组约500例,预计2026年公布结果。
3.3.2.4 ABBV-706(SEZ6-ADC)
癫痫相关同源蛋白6(seizure-related 6 homolog,SEZ6)是一种Ⅰ型跨膜蛋白,在SCLC与高级别神经内分泌肿瘤中高表达,正常组织低表达,成为肿瘤靶向治疗的理想靶点。ABBV-706通过靶向SEZ6将拓扑异构酶Ⅰ载荷递送至肿瘤细胞,具有稳定的连接子附着,药物抗体比为6。2025年WCLC公布其Ⅰ期临床试验结果[73],共入组80例SCLC患者(1.8 mg/kg组41例,2.5 mg/kg组39例)。中位既往治疗线数为2线(范围:1~6),中位治疗持续时间5.8个月(范围:0.7~11.3)。两剂量组ORR相近,但1.8 mg/kg组DoR和PFS更长。在未使用拓扑异构酶Ⅰ抑制剂及二线使用ABBV-706的患者中,1.8 mg/kg组ORR更高(分别为62.1%和81.3%)。1.8 mg/kg组和2.5 mg/kg组任意级别TRAE发生率分别为85%和95%,最常见为贫血(51% vs. 74%)和乏力(34% vs. 39%);≥3级TRAE发生率分别为49%和77%,以贫血(39% vs. 62%)和中性粒细胞减少(17% vs. 31%)为主。严重TRAE发生率分别为17%(1.8 mg/kg)和10%(2.5 mg/kg)。2025年9月已启动一线ES-SCLC的Ⅱ/Ⅲ期试验(NCT07155174),旨在评估ABBV-706联合PD-L1抑制剂阿替利珠单抗,对比标准疗法作为ES-SCLC一线治疗的疗效和安全性。
4肺神经内分泌肿瘤病理及生物学特征
肺神经内分泌肿瘤是一类具有多样生长模式、转移倾向、分子特征和治疗敏感性的异质性肿瘤。神经内分泌肿瘤可分为不同亚型,包括高分化、低级别典型类癌、高分化非典型类癌以及低分化神经内分泌癌,即高级别大细胞神经内分泌癌(large-cell neuroendocrine carcinoma,LCNEC)和SCLC[74]。目前除了肺神经内分泌肿瘤的标准组织病理学分类外,分子和测序技术的应用为理解这些疾病的生物学特性带来了新的进展。
4.1
基因组、转录组特征及分型
类癌以低突变负荷和染色质重塑基因改变的倾向为特征[75, 76]。吸烟相关的 G→C 替换频率高于 A→T 替换,且在SCLC中的 G→C 替换频率低于神经内分泌癌[77]。据报道,11%~22% 的类癌存在体细胞 MEN1 突变,且 MEN1 基因组改变和 MEN1 基因低表达均与不良预后相关[78, 79]。与SCLC(TP53 和 RB1 灭活突变率高)不同,肺类癌通常不携带这些突变[80]。
George 等于2018年描述了LCNEC的转录组特征,该研究对75例患者的LCNEC样本进行了基因组和转录组分析[81]。该研究定义了两个分子亚群:Ⅰ型LCNEC包括 TP53 和 STK11-KEAP1 改变,Ⅱ型LCNEC包括 TP53 和 RB1 激活。Ⅰ型组与SCLC共享转录组神经内分泌特征,包括ASCL1和DLL3的高表达以及NOTCH的低表达。Ⅱ型LCNEC组包括ASCL1和DLL3的低表达以及NOTCH的高表达。将这两种类型的转录组特征与其他肺肿瘤(包括SCLC、肺腺癌、类癌和鳞状细胞癌)进行了比较,发现LCNEC与SCLC最为相似。2022年世界卫生组织神经内分泌肿瘤分类特别提到了LCNEC的三种亚型:具有SCLC样特征的LCNEC、具有NSCLC特征的LCNEC和具有类癌样特征的LCNEC[82]。
此外,关于肺神经内分泌肿瘤已确定了具有不同临床特征的转录组亚群。其中一个亚群的特征是ASCL1过表达,主要与周围型类癌相关[83]。还描述了一个具有MEN1突变的ASCL1阴性亚群,以及第3个亚群,其肿瘤为支气管内型,主要发生在50岁以下的患者中。2019年,一项利用肺类癌和LCNEC的多组学数据集的研究揭示了肺类癌的4个不同分子群[84]。A组以典型类癌为主,ASCL1和DLL3过表达;A1组包括非典型类癌;B组富含免疫抑制性单核细胞和巨噬细胞,树突状细胞较少,且MEN1的体细胞突变增加;第4组是一类新的类癌肿瘤,与LCNEC具有相似特征,称为超类癌。超类癌患者的OS比其他类型的肺类癌患者更差。
以上的分子特性和分型可能影响未来肺神经内分泌肿瘤的分类和分期,进而改善辅助治疗或转移性疾病的管理指南。
4.2
治疗进展
肺类癌通常表达生长抑素受体。针对晚期肺神经内分泌肿瘤的第一项随机前瞻性研究(SPINET试验)由于入组不足而提前终止,但显示兰瑞肽治疗联合最佳支持治疗与安慰剂联合最佳支持治疗相比,PFS有所改善(16.6个月vs. 13.6个月)[85]。尽管生长抑素类似物未显示出优越性,但在随机Ⅱ期LUNA研究中,9个月PFS率高于依维莫司(帕瑞肽39.0%,依维莫司33.3%,联合治疗58.5%)[86]。对于生长抑素受体2阳性患者,生长抑素类似物是一种重要的治疗方式,因此通常是首选的一线治疗选择,尤其是对于典型类癌[87, 88]。
卡博替尼可抑制多种受体酪氨酸激酶,包括c-MET和VEGFR-2,这对神经内分泌肿瘤的发病机制至关重要[89]。一项Ⅲ期研究评估了卡博替尼(与安慰剂相比)在先前接受过全身治疗后进展的晚期胰腺或胰腺外神经内分泌肿瘤(包括肺类癌)患者中的疗效,结果显示卡博替尼治疗组患者的PFS有所改善。在所有胰腺外神经内分泌肿瘤的合并数据中,卡博替尼组的中位PFS为8.4 个月(95%CI 7.6~12.7),安慰剂组为3.9个月(95%CI 3.0~5.7)[90]。这些数据可能会被纳入高分化肺神经内分泌肿瘤的治疗指南[91]。此外,目前多项研究正在探索卡博替尼与生长抑素类似物和替莫唑胺的联合使用。CABOTEM是一项Ⅱ期临床试验,评估卡博替尼联合替莫唑胺在接受依维莫司、舒尼替尼或肽受体放射性核素治疗后进展的肺和胃肠道神经内分泌肿瘤患者中的活性和安全性(NCT04893785)。另一种策略是使用β射线(177Lu)或α射线(212Pb和225Ac)发射体的肽受体放射性核素治疗,靶向表达生长抑素受体2的肺神经内分泌肿瘤。225Ac(RYZ101)正在先前接受过肽受体放射性核素治疗后进展的生长抑素受体2阳性胃肠道神经内分泌肿瘤中进行研究(NCT05477576)。基于225Ac的肽受体放射性核素治疗也可能对高分化生长抑素受体2阳性肺类癌有效。
5转化性SCLC进展
转化性SCLC作为SCLC的一种特殊类型,在发病机制、临床特征、治疗策略和预后方面均与原发性SCLC有所不同。目前转化性SCLC尚无标准治疗方案,主要参照原发性SCLC治疗,但治疗疗效仍差强人意。
5.1
分子分型
与原发性SCLC一致,转化性SCLC也可分为A型(ASCL1高表达)、N型(NEUROD1高表达)、P型(POU2F3高表达)和Y型(YAP1高表达)[92, 93]。我们团队基于DNA测序数据,将EGFR突变转化性SCLC分为RB1突变型和RB1野生型,两种分型具有不同的转化时间、PI3K/AKT通路活性和免疫微环境特征[94]。同时,基于全转录组数据,利用无监督主成分分析将转化性SCLC分为:LUAD样亚型,呈现与经典肺腺癌趋于一致的转录组特征;非LUAD样亚型,紧密关联于典型SCLC分子表型[95]。
5.2
治疗策略5.2.1 ICIs联合化疗
回顾性数据表明,大多数转化性SCLC患者接受了EC方案化疗[96, 97],而部分患者接受ICIs治疗取得了良好的生存效果[98, 99]。目前免疫联合化疗已成为原发性SCLC的一线标准治疗,其在转化性SCLC中的疗效仍在探索。
Thomas牵头的一项Ⅱ期研究(NCT04538378)正在评估度伐利尤单抗联合奥拉帕利治疗EGFR突变型NSCLC转化为SCLC或其他神经内分泌肿瘤患者的疗效。我们一项回顾性分析显示,免疫联合化疗±抗血管生成治疗虽然在转化性SCLC患者中PFS方面未见明显优势,但OS显著延长[100]。同时,本团队设计开展了一项斯鲁利单抗联合化疗治疗转化性SCLC的前瞻性Ⅱ期临床研究(NCT05957510),目前正在入组阶段[101],期待结果的公布。考虑到ICIs与化疗、抗血管生成治疗等其他治疗策略分别在EGFR突变型腺癌和经典SCLC中的作用,将ICIs与这些治疗策略联合使用可能为SCLC转化提供新的治疗前景。
5.2.2 EGFR- TKI联合化疗
研究表明,发生SCLC转化的EGFR突变型腺癌患者可能从化疗联合EGFR-酪氨酸激酶抑制剂(tyrosine kinase inhibitor,TKI)治疗中获益。多项随机临床试验观察到化疗联合EGFR-TKI治疗具有显著的生存优势。例如,在Lai等的一项研究中,两例EGFR突变型腺癌患者最初接受第一代TKI治疗,随后接受奥希替尼治疗,最终转化为携带EGFR第19外显子缺失的SCLC,且对EGFR-TKI耐药。研究发现,SCLC转化后,接受厄洛替尼联合EP方案治疗的患者比仅接受EP化疗的患者具有更长的PFS[102]。在Yang等的另一项研究中,对7例二次活检证实从腺癌转化为SCLC的患者进行了研究,其中1例携带持续EGFR突变的患者在SCLC转化后继续接受TKI治疗,PFS达到12个月。该病例表明,化疗联合EGFR-TKI维持治疗可能对部分从EGFR突变型腺癌转化为SCLC的患者有效,提示EGFR-TKI对某些转化型SCLC具有治疗效果。有趣的是,尽管联合治疗在PFS方面有明显获益,但并未给转化型SCLC患者带来OS获益。这可能反映了转化后SCLC成分占主导地位,而剩余的少量腺癌成分可能通过EGFR-TKI治疗获得微小获益。转化型SCLC患者的OS可能在很大程度上取决于对SCLC成分的治疗效果[103]。
5.3
新药探索5.3.1 BCL-2抑制剂
BCL-2抑制剂ABT-263已被证实对部分SCLC患者有效[13],因此可能对转化性SCLC患者也有效。Niederst等的研究显示,转化为SCLC的EGFR突变细胞对ABT-263高度敏感,且比对EGFR-TKI耐药的T790M耐药突变NSCLC细胞系更敏感[104]。然而,仍需要更多临床试验来探索其疗效和安全性。
5.3.2 靶向DNA损伤检查点
一种治疗选择是靶向由RB1缺失引起的细胞周期脆弱性。癌细胞中RB1缺失会对DNA复制机制造成压力,从而增强癌细胞对检查点激酶(checkpoint kinase,CHK)、polo样激酶(polo-like kinase,PLK)抑制剂的敏感性[105, 106]。因此,CHK、PLK抑制剂通过靶向RB1等DNA损伤检查点,极有可能有效治疗转化型SCLC。最近的研究还表明,AURK是转化性SCLC的潜在治疗靶点。有报道称,EGFR-TKI会激活RB1缺陷细胞中的AURK,从而抑制其诱导的凋亡[107]。在这种情况下,将AURKA抑制剂与EGFR-TKI联合使用可以对抗EGFR-TKI耐药性,从而阻止SCLC转化。
5.3.3 表观遗传治疗
靶向细胞可塑性可能是对抗SCLC转化的一种方法,这将增强NSCLC患者对靶向治疗的反应[108]。靶向细胞可塑性的有效手段包括抑制染色质修饰酶EZH2。RB1缺失通过升高EZH2(PRC2的酶催化亚基)促进前列腺癌细胞的谱系转化。此外,在原发性SCLC中也可检测到EZH2蛋白的过表达[109]。经典SCLC通常具有上调的EZH2表达,EZH2抑制剂已被用于SCLC的临床试验[110]。因此,EZH2抑制剂可能是治疗转化型SCLC患者的一种可行选择。对于EGFR/TP53/RB1三重突变、SCLC转化风险相对较高的肺癌患者,已尝试EZH2抑制剂与EGFR-TKI联合治疗。
6展望
迄今为止,靶向治疗在SCLC中的应用未能取得成功,且免疫治疗在NSCLC中的成功尚未在这种恶性肿瘤中完全体现。SCLC治疗手段缺乏突破性进展,主要是由于其高度的肿瘤可塑性和临床试验入组时患者群体未经过筛选。因此,根据患者的主要分子亚型和特定蛋白水平改变对患者进行分层,可能有助于为这种难治性疾病开发新的靶向治疗策略。同时,由于SCLC很少进行手术,且小活检标本往往无法反映整个肿瘤的表达谱,因此分子状态的诊断可能具有挑战性,对基于血液的肿瘤成分(如循环肿瘤细胞、循环肿瘤DNA和肿瘤来源的细胞外囊泡)的分析,可为监测疾病过程中的分子表型以及评估治疗反应和预后的生物标志物提供替代机会。
【关键词】小细胞肺癌;分子分型;新型疗法;抗体偶联药物;免疫治疗
【Keywords】small-cell lung cancer;molecular subtype;novel therapies;antibody-drug conjugate;immunotherapy
【中图分类号】R734.2
【文献标识码】A
DOI: 10.12019/j.issn.1671-5144.202601045
基金项目:国家自然科学基金(82373349);广州市科技计划项目(2025A03J4506);广东省肺癌转化医学重点实验室项目(2017B030314120)。
通讯作者:吴一龙,E-mail:syylwu@live.cn。
参考文献:
[1]
BARNARD W G. The nature of the “oat-celled sarcoma” of the mediastinum[J]. J Pathol Bacteriol, 1926, 29(3): 241−244. doi: 10.1002/path.1700290304.
[2]
AZZOPARDI J G. Oat-cell carcinoma of the bronchus[J]. J Pathol Bacteriol, 1959, 78(2): 513−519. doi: 10.1002/path.1700780218.
[3]
GEORGE J, LIM J S, JANG S J, et al. Comprehensive genomic profiles of small cell lung cancer[J]. Nature, 2015, 524(7563): 47−53. doi: 10.1038/nature14664.
[4]
SUTHERLAND K D, PROOST N, BROUNS I, et al. Cell of origin of small cell lung cancer: inactivation of Trp53 and Rb1 in distinct cell types of adult mouse lung[J]. Cancer Cell, 2011, 19(6): 754−764. doi: 10.1016/j.ccr.2011.04.019.
[5]
PEIFER M, FERNÁNDEZ-CUESTA L, SOS M L, et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer[J]. Nat Genet, 2012, 44(10): 1104−1110. doi: 10.1038/ng.2396.
[6]
LIM J S, IBASETA A, FISCHER M M, et al. Intratumoural heterogeneity generated by Notch signalling promotes small-cell lung cancer[J]. Nature, 2017, 545(7654): 360−364. doi: 10.1038/nature22323.
[7]
CARNEY D N, GAZDAR A F, BEPLER G, et al. Establishment and identification of small cell lung cancer cell lines having classic and variant features[J]. Cancer Res, 1985, 45(6): 2913−2923. doi: 10.1016/s0169-5002(86)80023-x.
[8]
POIRIER J T, DOBROMILSKAYA I, MORIARTY W F, et al. Selective tropism of Seneca Valley virus for variant subtype small cell lung cancer[J]. J Natl Cancer Inst, 2013, 105(14): 1059−1065. doi: 10.1093/jnci/djt130.
[9]
BORROMEO M D, SAVAGE T K, KOLLIPARA R K, et al. ASCL1 and NEUROD1 reveal heterogeneity in pulmonary neuroendocrine tumors and regulate distinct genetic programs[J]. Cell Rep, 2016, 16(5): 1259−1272. doi: 10.1016/j.celrep.2016.06.081.
[10]
MCCOLL K, WILDEY G, SAKRE N, et al. Reciprocal expression of INSM1 and YAP1 defines subgroups in small cell lung cancer[J]. Oncotarget, 2017, 8(43): 73745−73756. doi: 10.18632/oncotarget.20572.
[11]
HUANG Y H, KLINGBEIL O, HE X Y, et al. POU2F3 is a master regulator of a tuft cell-like variant of small cell lung cancer[J]. Genes Dev, 2018, 32(13-14): 915−928. doi: 10.1101/gad.314815.118.
[12]
RUDIN C M, POIRIER J T, BYERS L A, et al. Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data[J]. Nat Rev Cancer, 2019, 19(5): 289−297. doi: 10.1038/s41568-019-0133-9.
[13]
GAY C M, STEWART C A, PARK E M, et al. Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities[J]. Cancer Cell, 2021, 39(3): 346-360. e7. doi: 10.1016/j.ccell.2020.12.014.
[14]
LIU Q, ZHANG J, GUO C C, et al. Proteogenomic characterization of small cell lung cancer identifies biological insights and subtype-specific therapeutic strategies[J]. Cell, 2024, 187(1): 184-203. e28, doi: 10.1016/j.cell.2023.12.004.
[15]
NABET B Y, HAMIDI H, LEE M C, et al. Immune heterogeneity in small-cell lung cancer and vulnerability to immune checkpoint blockade[J]. Cancer Cell, 2024, 42(3): 429-443. e4, doi: 10.1016/j.ccell.2024.01.010.
[16]
PARK S, HONG T H, HWANG S, et al. Comprehensive analysis of transcription factor-based molecular subtypes and their correlation to clinical outcomes in small-cell lung cancer[J]. eBioMedicine, 2024, 102: 105062. doi: 10.1016/j.ebiom.2024.105062.
[17]
HUO Z T, DUAN Y Q, ZHAN D D, et al. Proteomic stratification of prognosis and treatment options for small cell lung cancer[J]. Genomics Proteomics Bioinformatics, 2024, 22(2): qzae033. doi: 10.1093/gpbjnl/qzae033.
[18]
CHEN H Q, DENG C Q, GAO J, et al. Integrative spatial analysis reveals tumor heterogeneity and immune colony niche related to clinical outcomes in small cell lung cancer[J]. Cancer Cell, 2025, 43(3): 519-536. e5, doi: 10.1016/j.ccell.2025.01.012.
[19]
FOX W, SCADDING J G. Medical Research Council comparative trial of surgery and radiotherapy for primary treatment of small-celled or oat-celled carcinoma of bronchus. Ten-year follow-up[J]. Lancet, 1973, 2(7820): 63−65. doi: 10.1016/s0140-6736(73)93260-1.
[20]
LAD T, PIANTADOSI S, THOMAS P, et al. A prospective randomized trial to determine the benefit of surgical resection of residual disease following response of small cell lung cancer to combination chemotherapy[J]. Chest, 1994, 106(6S): 320S−323S. doi: 10.1378/chest.106.6_supplement.320s.
[21]
SHEPHERD F A, GINSBERG R J, FELD R, et al. Surgical treatment for limited small-cell lung cancer. The University of Toronto Lung Oncology Group experience[J]. J Thorac Cardiovasc Surg, 1991, 101(3): 385−393.
[22]
TSUCHIYA R, SUZUKI K, ICHINOSE Y, et al. Phase II trial of postoperative adjuvant cisplatin and etoposide in patients with completely resected stage I-IIIa small cell lung cancer: the Japan Clinical Oncology Lung Cancer Study Group Trial (JCOG9101)[J]. J Thorac Cardiovasc Surg, 2005, 129(5): 977−983. doi: 10.1016/j.jtcvs.2004.05.030.
[23]
LIU T T, CHEN Z H, DANG J, et al. The role of surgery in stage I to III small cell lung cancer: a systematic review and meta-analysis[J]. PLoS One, 2018, 13(12): e0210001. doi: 10.1371/journal.pone.0210001.
[24]
COMBS S E, HANCOCK J G, BOFFA D J, et al. Bolstering the case for lobectomy in stages I, II, and IIIA small-cell lung cancer using the National Cancer Data Base[J]. J Thorac Oncol, 2015, 10(2): 316−323. doi: 10.1097/JTO.0000000000000402.
[25]
YANG C F J, CHAN D Y, SPEICHER P J, et al. Role of adjuvant therapy in a population-based cohort of patients with early-stage small-cell lung cancer[J]. J Clin Oncol, 2016, 34(10): 1057−1064. doi: 10.1200/JCO.2015.63.8171.
[26]
PIGNON J P, TRIBODET H, SCAGLIOTTI G V, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group[J]. J Clin Oncol, 2008, 26(21): 3552−3559. doi: 10.1200/JCO.2007.13.9030.
[27]
RAMI-PORTA R, WITTEKIND C, GOLDSTRAW P. Complete resection in lung cancer surgery: proposed definition[J]. Lung Cancer, 2005, 49(1): 25−33. doi: 10.1016/j.lungcan.2005.01.001.
[28]
EDWARDS J G, CHANSKY K, VAN SCHIL P, et al. The IASLC lung cancer staging project: analysis of resection margin status and proposals for residual tumor descriptors for non-small cell lung cancer[J]. J Thorac Oncol, 2020, 15(3): 344−359. doi: 10.1016/j.jtho.2019.10.019.
[29]
VARLOTTO J M, RECHT A, FLICKINGER J C, et al. Lobectomy leads to optimal survival in early-stage small cell lung cancer: a retrospective analysis[J]. J Thorac Cardiovasc Surg, 2011, 142(3): 538−546. doi: 10.1016/j.jtcvs.2010.11.062.
[30]
RAMI-PORTA R, WITTEKIND C, GOLDSTRAW P. Complete resection in lung cancer surgery: from definition to validation and beyond[J]. J Thorac Oncol, 2020, 15(12): 1815−1818. doi: 10.1016/j.jtho.2020.09.006.
[31]
LIU J C, WANG L M, SHU W B, et al. Safety and effectiveness of neoadjuvant immunotherapy combined with chemotherapy followed by surgical resection in patients with stage I-IIIA small-cell lung cancer: a retrospective single-arm clinical trial[J]. J Thorac Dis, 2022, 14(11): 4405−4415. doi: 10.21037/jtd-22-1287.
[32]
DUAN H T, SHI L, SHAO C J, et al. A multicenter, single-arm, open study of neoadjuvant or conversion atezolizumab in combination with chemotherapy in resectable small cell lung cancer (cohort study)[J]. Int J Surg, 2023, 109(9): 2641−2649. doi: 10.1097/JS9.0000000000000501.
[33]
ZHU L H, LIU J C, HUANG X H, et al. Preoperative immunochemotherapy versus chemotherapy as first-line treatment for patients with stage I-IIIB small-cell lung cancer[J]. BMC Cancer, 2025, 25(1): 8. doi: 10.1186/s12885-024-13405-0.
[34]
ZHAO Z C, RONG H, LI M X, et al. Clinical outcomes for neoadjuvant immunochemotherapy or chemotherapy versus concurrent chemoradiotherapy in limited-stage small-cell lung cancer: a retrospective, multi-center, real-world study[J]. Int J Cancer, 2026, 158(4): 1008−1020. doi: 10.1002/ijc.70153.
[35]
SUN F H, ZHANG L L, SUN L D, et al. Surgery after induced anti-PD-L1 therapy and chemotherapy for stage I‒III small-cell lung cancer: a phase 2 trial (LungMate-005)[J]. Cell Discov, 2025, 11(1): 95. doi: 10.1038/s41421-025-00838-5.
[36]
HUANG C, CHEN Z Y, XU Z Y, et al. P3.13. 33 clinical outcomes and single-cell map of neoadjuvant immunochemotherapy for stage II-IIIb LS-SCLC: a real-world study[J]. J Thorac Oncol, 2025, 20(10): S519−S520. doi: 10.1016/j.jtho.2025.09.969.
[37]
PIGNON J P, ARRIAGADA R, IHDE D C, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer[J]. N Engl J Med, 1992, 327(23): 1618−1624. doi: 10.1056/NEJM199212033272302.
[38]
WARDE P, PAYNE D. Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis[J]. J Clin Oncol, 1992, 10(6): 890−895. doi: 10.1200/JCO.1992.10.6.890.
[39]
CHENG Y, SPIGEL D R, CHO B C, et al. Durvalumab after chemoradiotherapy in limited-stage small-cell lung cancer[J]. N Engl J Med, 2024, 391(14): 1313−1327. doi: 10.1056/NEJMoa2404873.
[40]
GRONBERG B H, AANERUD M, DUMOULIN D W, et al. Randomized phase II trial investigating whether atezolizumab after chemoradiotherapy (CRT) prolongs survival in limited stage (LS) small cell lung cancer (SCLC)[J]. J Clin Oncol, 2025, 43(17S): LBA8005. doi: 10.1200/jco.2025.43.17_suppl.lba8005.
[41]
HIGGINS K A, HU C, ROSS H J, et al. Chemoradiation ± atezolizumab in limited-stage small cell lung cancer: results of NRG oncology/alliance LU005[J]. J Clin Oncol, 2026: JCO2501569. doi: 10.1200/JCO-25-01569. Online ahead of print.
[42]
BRADLEY J D, SUGAWARA S, LEE K H, et al. Simultaneous durvalumab and platinum-based chemoradiotherapy in unresectable stage III non-small cell lung cancer: the phase III PACIFIC-2 study[J]. J Clin Oncol, 2025, 43(33): 3610−3621. doi: 10.1200/JCO-25-00036.
[43]
DE RUYSSCHER D, RAMALINGAM S, URBANIC J, et al. CheckMate 73L: a phase 3 study comparing nivolumab plus concurrent chemoradiotherapy followed by nivolumab with or without ipilimumab versus concurrent chemoradiotherapy followed by durvalumab for previously untreated, locally advanced stage III non-small-cell lung cancer[J]. Clin Lung Cancer, 2022, 23(3): e264−e268. doi: 10.1016/j.cllc.2021.07.005.
[44]
JABBOUR S K, HOUGHTON B, ROBINSON A G, et al. KEYNOTE-867: phase 3, randomized, placebo-controlled study of stereotactic body radiotherapy (SBRT) with or without pembrolizumab in patients with unresected stage I or II non–small-cell lung cancer (NSCLC)[J]. Int J Radiat Oncol Biol Phys, 2022, 114(3S): e376−e377. doi: 10.1016/j.ijrobp.2022.07.1516.
[45]
YU J Y, JIANG L L, ZHAO L N, et al. High-dose hyperfractionated simultaneous integrated boost radiotherapy versus standard-dose radiotherapy for limited-stage small-cell lung cancer in China: a multicentre, open-label, randomised, phase 3 trial[J]. Lancet Respir Med, 2024, 12(10): 799−809. doi: 10.1016/S2213-2600(24)00189-9.
[46]
GRØNBERG B H, KILLINGBERG K T, FLØTTEN Ø, et al. High-dose versus standard-dose twice-daily thoracic radiotherapy in limited-stage SCLC: final survival data, long-term toxicity, and relapse patterns in a randomized, open-label, phase II trial[J]. J Thorac Oncol, 2025, 20(8): 1108−1119. doi: 10.1016/j.jtho.2025.04.007.
[47]
CHEN D W, ZOU B, LI B T, et al. Adebrelimab plus chemotherapy and sequential thoracic radiotherapy as first-line therapy for extensive-stage small-cell lung cancer (ES-SCLC): a phase II trial[J]. eClinicalMedicine, 2024, 75: 102795. doi: 10.1016/j.eclinm.2024.102795.
[48]
AIZER A A, TANGUTURI S K, SHI D D, et al. Stereotactic radiosurgery in patients with small cell lung cancer and 1-10 brain metastases: a multi-institutional, phase II, prospective clinical trial[J]. J Clin Oncol, 2025, 43(27): 2986−2997. doi: 10.1200/JCO-25-00056.
[49]
DINGEMANS A M C, FRÜH M, ARDIZZONI A, et al. Small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up[J]. Ann Oncol, 2021, 32(7): 839−853. doi: 10.1016/j.annonc.2021.03.207.
[50]
RUDIN C M, BRAMBILLA E, FAIVRE-FINN C, et al. Small-cell lung cancer[J]. Nat Rev Dis Primers, 2021, 7(1): 3. doi: 10.1038/s41572-020-00235-0.
[51]
RUANO-RAVIÑA A, PROVENCIO-PULLA M, PÉREZ-RÍOS M. Small cell lung cancer-a neglected disease with more data needed[J]. JAMA Netw Open, 2022, 5(3): e224837. doi: 10.1001/jamanetworkopen.2022.4837.
[52]
HORN L, MANSFIELD A S, SZCZĘSNA A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer[J]. N Engl J Med, 2018, 379(23): 2220−2229. doi: 10.1056/NEJMoa1809064.
[53]
PAZ-ARES L, DVORKIN M, CHEN Y B, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial[J]. Lancet, 2019, 394(10212): 1929−1939. doi: 10.1016/S0140-6736(19)32222-6.
[54]
OWONIKOKO T K, PARK K, GOVINDAN R, et al. Nivolumab and ipilimumab as maintenance therapy in extensive-disease small-cell lung cancer: CheckMate 451[J]. J Clin Oncol, 2021, 39(12): 1349−1359. doi: 10.1200/JCO.20.02212.
[55]
RUDIN C M, AWAD M M, NAVARRO A, et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, phase III KEYNOTE-604 study[J]. J Clin Oncol, 2020, 38(21): 2369−2379. doi: 10.1200/JCO.20.00793.
[56]
PAZ-ARES L, BORGHAEI H, LIU S V, et al. Efficacy and safety of first-line maintenance therapy with lurbinectedin plus atezolizumab in extensive-stage small-cell lung cancer (IMforte): a randomised, multicentre, open-label, phase 3 trial[J]. Lancet, 2025, 405(10495): 2129−2143. doi: 10.1016/S0140-6736(25)01011-6.
[57]
PAULSON K G, LAU S C M, AHN M J, et al. Safety and activity of tarlatamab in combination with a PD-L1 inhibitor as first-line maintenance therapy after chemo-immunotherapy in patients with extensive-stage small-cell lung cancer (DeLLphi-303): a multicentre, non-randomised, phase 1b study[J]. Lancet Oncol, 2025, 26(10): 1300−1311. doi: 10.1016/S1470-2045(25)00480-2.
[58]
WERMKE M, LAU S, MOSKOVITZ M T, et al. 2757O Tarlatamab with first-line chemoimmunotherapy for extensive stage small cell lung cancer (ES-SCLC): DeLLphi-303 study[J]. Ann Oncol, 2025, 36(2S): S1366. doi: 10.1016/j.annonc.2025.08.3368.
[59]
VON PAWEL J, SCHILLER J H, SHEPHERD F A, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer[J]. J Clin Oncol, 1999, 17(2): 658−667. doi: 10.1200/JCO.1999.17.2.658.
[60]
VON PAWEL J, JOTTE R, SPIGEL D R, et al. Randomized phase III trial of amrubicin versus topotecan as second-line treatment for patients with small-cell lung cancer[J]. J Clin Oncol, 2014, 32(35): 4012−4019. doi: 10.1200/JCO.2013.54.5392.
[61]
MOUNTZIOS G, SUN L H, CHO B C, et al. Tarlatamab in small-cell lung cancer after platinum-based chemotherapy[J]. N Engl J Med, 2025, 393(4): 349−361. doi: 10.1056/NEJMoa2502099.
[62]
TRIGO J, SUBBIAH V, BESSE B, et al. Lurbinectedin as second-line treatment for patients with small-cell lung cancer: a single-arm, open-label, phase 2 basket trial[J]. Lancet Oncol, 2020, 21(5): 645−654. doi: 10.1016/S1470-2045(20)30068-1.
[63]
AHN M J, CHO B C, FELIP E, et al. Tarlatamab for patients with previously treated small-cell lung cancer[J]. N Engl J Med, 2023, 389(22): 2063−2075. doi: 10.1056/NEJMoa2307980.
[64]
ELEZ M E, TABERNERO J, GEARY D, et al. First-in-human phase I study of lurbinectedin (PM01183) in patients with advanced solid tumors[J]. Clin Cancer Res, 2014, 20(8): 2205−2214. doi: 10.1158/1078-0432.CCR-13-1880.
[65]
AIX S P, CIULEANU T E, NAVARRO A, et al. Combination lurbinectedin and doxorubicin versus physician's choice of chemotherapy in patients with relapsed small-cell lung cancer (ATLANTIS): a multicentre, randomised, open-label, phase 3 trial[J]. Lancet Respir Med, 2023, 11(1): 74−86. doi: 10.1016/S2213-2600(22)00309-5.
[66]
CHENG Y, WU C J, WU L, et al. A pivotal bridging study of lurbinectedin as second-line therapy in Chinese patients with small cell lung cancer[J]. Sci Rep, 2024, 14(1): 3598. doi: 10.1038/s41598-024-54223-5.
[67]
王启鸣, 石建华, 柴小立, 等. ZG006(Alveltamig)在晚期小细胞肺癌患者中的耐受性、安全性、有效性和药代动力学的剂量递增和多队列扩展的II期临床研究(ZG006-001)[J]. 临床肿瘤学杂志, 2025(CSCO年会专刊): 138−139.//第二十八届中国临床肿瘤学会(CSCO)学术年会专刊.
[68]
WERMKE M, GAMBARDELLA V, KUBOKI Y, et al. Phase I dose-escalation results for the delta-like ligand 3/CD3 IgG-like T-cell engager obrixtamig (BI 764532) in patients with delta-like ligand 3+ small cell lung cancer or neuroendocrine carcinomas[J]. J Clin Oncol, 2025, 43(27): 3021−3031. doi: 10.1200/JCO-25-00363.
[69]
American Association for Cancer Research. Investigational antibody-drug conjugate shows clinical benefit against previously treated small cell lung cancer[EB/OL]. (2025-10-22) [2026-01-01]. https://www.aacr.org/about-the-aacr/newsroom/news-releases/investigational-antibody-drug-conjugate-shows-clinical-benefit-against-previously-treated-small-cell-lung-cancer/.
[70]
KOGAWA T, MITANI S, BERZ D, et al. IDeate-PanTumor02: a phase 1b/2 study to evaluate the efficacy and safety of ifinatamab deruxtecan (I-DXd) in patients (pts) with recurrent or metastatic solid tumors[J]. J Clin Oncol, 2025, 43(16S): TPS3157. doi: 10.1200/JCO.2025.43.16_suppl.TPS3157.
[71]
RUDIN C M, JOHNSON M L, PAZ-ARES L, et al. Ifinatamab deruxtecan in patients with extensive-stage small cell lung cancer: primary analysis of the phase II IDeate-Lung01 trial[J]. J Clin Oncol, 2025, doi: 10.1200/JCO-25-02142. Online ahead of print.
[72]
DOWLATI A, CHIANG A C, CERVANTES A, et al. Phase 2 open-label study of sacituzumab govitecan as second-line therapy in patients with extensive-stage SCLC: results from TROPiCS-03[J]. J Thorac Oncol, 2025, 20(6): 799−808. doi: 10.1016/j.jtho.2024.12.028.
[73]
BYERS L A, CHO B C, COOPER A, et al. OA06.04 Safety and efficacy of ABBV-706, a seizure-related homolog protein 6-targeting antibody-drug conjugate, in R/R SCLC[J]. J Thorac Oncol, 2025, 20(10 Suppl 1): S23, doi: 10.1016/j.jtho.2025.09.049.
[74]
HENDIFAR A E, MARCHEVSKY A M, TULI R. Neuroendocrine tumors of the lung: current challenges and advances in the diagnosis and management of well-differentiated disease[J]. J Thorac Oncol, 2017, 12(3): 425−436. doi: 10.1016/j.jtho.2016.11.2222.
[75]
FERNANDEZ-CUESTA L, PEIFER M, LU X, et al. Frequent mutations in chromatin-remodelling genes in pulmonary carcinoids[J]. Nat Commun, 2014, 5: 3518. doi: 10.1038/ncomms4518.
[76]
CENTONZE G, BIGANZOLI D, PRINZI N, et al. Beyond traditional morphological characterization of lung neuroendocrine neoplasms: in silico study of next-generation sequencing mutations analysis across the four World Health Organization defined groups[J]. Cancers (Basel), 2020, 12(10): 2753. doi: 10.3390/cancers12102753.
[77]
REKHTMAN N, PIETANZA M C, HELLMANN M D, et al. Next-generation sequencing of pulmonary large cell neuroendocrine carcinoma reveals small cell carcinoma-like and non-small cell carcinoma-like subsets[J]. Clin Cancer Res, 2016, 22(14): 3618−3629. doi: 10.1158/1078-0432.CCR-15-2946.
[78]
SWARTS D R A, SCARPA A, CORBO V, et al. MEN1 gene mutation and reduced expression are associated with poor prognosis in pulmonary carcinoids[J]. J Clin Endocrinol Metab, 2014, 99(2): E374−E378. doi: 10.1210/jc.2013-2782.
[79]
BARTSCH D K, ALBERS M B, LOPEZ C L, et al. Bronchopulmonary neuroendocrine neoplasms and their precursor lesions in multiple endocrine neoplasia type 1[J]. Neuroendocrinology, 2016, 103(3-4): 240−247. doi: 10.1159/000435921.
[80]
BEASLEY M B, LANTUEJOUL S, ABBONDANZO S, et al. The P16/cyclin D1/Rb pathway in neuroendocrine tumors of the lung[J]. Hum Pathol, 2003, 34(2): 136−142. doi: 10.1053/hupa.2003.8.
[81]
GEORGE J, WALTER V, PEIFER M, et al. Integrative genomic profiling of large-cell neuroendocrine carcinomas reveals distinct subtypes of high-grade neuroendocrine lung tumors[J]. Nat Commun, 2018, 9(1): 1048. doi: 10.1038/s41467-018-03099-x.
[82]
RINDI G, METE O, UCCELLA S, et al. Overview of the 2022 WHO classification of neuroendocrine neoplasms[J]. Endocr Pathol, 2022, 33(1): 115−154. doi: 10.1007/s12022-022-09708-2.
[83]
LADDHA S V, DA SILVA E M, ROBZYK K, et al. Integrative genomic characterization identifies molecular subtypes of lung carcinoids[J]. Cancer Res, 2019, 79(17): 4339−4347. doi: 10.1158/0008-5472.CAN-19-0214.
[84]
ALCALA N, LEBLAY N, GABRIEL A A G, et al. Integrative and comparative genomic analyses identify clinically relevant pulmonary carcinoid groups and unveil the supra-carcinoids[J]. Nat Commun, 2019, 10(1): 3407. doi: 10.1038/s41467-019-11276-9.
[85]
BAUDIN E, CAPDEVILA J, HÖRSCH D, et al. Treatment of advanced BP-NETS with lanreotide autogel/depot vs placebo: the phase III SPINET study[J]. Endocr Relat Cancer, 2024, 31(9): e230337. doi: 10.1530/erc-23-0337.
[86]
FEROLLA P, BRIZZI M P, MEYER T, et al. Efficacy and safety of long-acting pasireotide or everolimus alone or in combination in patients with advanced carcinoids of the lung and thymus (LUNA): an open-label, multicentre, randomised, phase 2 trial[J]. Lancet Oncol, 2017, 18(12): 1652−1664. doi: 10.1016/S1470-2045(17)30681-2.
[87]
BONGIOVANNI A, RECINE F, CELLI M, et al. Osteoblastic bone metastases from neuroendocrine tumor (NET) of unknown origin detected by 18fluorocholine PET/CT and its comparison with 68gallium-DOTATOC PET/CT: case report and review of the literature[J]. Medicine (Baltimore), 2017, 96(46): e8567. doi: 10.1097/md.0000000000008567.
[88]
SULLIVAN I, LE TEUFF G, GUIGAY J, et al. Antitumour activity of somatostatin analogues in sporadic, progressive, metastatic pulmonary carcinoids[J]. Eur J Cancer, 2017, 75: 259−267. doi: 10.1016/j.ejca.2016.11.034.
[89]
CHAN J A, FARIS J E, MURPHY J E, et al. Phase II trial of cabozantinib in patients with carcinoid and pancreatic neuroendocrine tumors (pNET)[J]. J Clin Oncol, 2017, 35(4S): 228. doi: 10.1200/jco.2017.35.4_suppl.228.
[90]
CHAN J A, GEYER S, ZEMLA T, et al. Phase 3 trial of cabozantinib to treat advanced neuroendocrine tumors[J]. N Engl J Med, 2025, 392(7): 653−665. doi: 10.1056/NEJMoa2403991.
[91]
GRANBERG D, ERIKSSON B, WILANDER E, et al. Experience in treatment of metastatic pulmonary carcinoid tumors[J]. Ann Oncol, 2001, 12(10): 1383−1391. doi: 10.1023/A:1012569909313.
[92]
QUINTANAL-VILLALONGA A, TANIGUCHI H, ZHAN Y A, et al. Multiomic analysis of lung tumors defines pathways activated in neuroendocrine transformation[J]. Cancer Discov, 2021, 11(12): 3028−3047. doi: 10.1158/2159-8290.CD-20-1863.
[93]
OH S, KOH J, KIM T M, et al. Transcriptomic heterogeneity of EGFR-mutant non-small cell lung cancer evolution toward small-cell lung cancer[J]. Clin Cancer Res, 2024, 30(20): 4729−4742. doi: 10.1158/1078-0432.CCR-24-0160.
[94]
HUANG J, ZHANG S L, ZHANG C, et al. Genotyping of RB1 status identifies two distinct subtypes in EGFR-mutant lung cancers with SCLC transformation[J]. Clin Transl Med, 2024, 14(5): e1683. doi: 10.1002/ctm2.1683.
[95]
SUN H, ZHANG C Y, ZHANG X H, et al. Transcriptomic analysis of transformed small-cell lung cancer from EGFR-mutated lung adenocarcinoma reveals distinct subgroups and precision therapy opportunities[J]. Biomark Res, 2025, 13(1): 79. doi: 10.1186/s40364-025-00789-9.
[96]
IAMS W T, BECKERMANN K E, ALMODOVAR K, et al. Small cell lung cancer transformation as a mechanism of resistance to PD-1 therapy in KRAS-mutant lung adenocarcinoma: a report of two cases[J]. J Thorac Oncol, 2019, 14(3): e45−e48. doi: 10.1016/j.jtho.2018.11.031.
[97]
OKEYA K, KAWAGISHI Y, MURANAKA E, et al. Hyperprogressive disease in lung cancer with transformation of adenocarcinoma to small-cell carcinoma during pembrolizumab therapy[J]. Intern Med, 2019, 58(22): 3295−3298. doi: 10.2169/internalmedicine.2892-19.
[98]
BAR J, OFEK E, BARSHACK I, et al. Transformation to small cell lung cancer as a mechanism of resistance to immunotherapy in non-small cell lung cancer[J]. Lung Cancer, 2019, 138: 109−115. doi: 10.1016/j.lungcan.2019.09.025.
[99]
LIU H, CHEN L H, ZHANG Z H, et al. Histomorphological transformation from non-small cell lung carcinoma to small cell lung carcinoma after targeted therapy or immunotherapy: a report of two cases[J]. Front Oncol, 2022, 12: 1022705. doi: 10.3389/fonc.2022.1022705.
[100]
ZHANG C Y, SUN H, SU J W, et al. A potential treatment option for transformed small-cell lung cancer on PD-L1 inhibitor-based combination therapy improved survival[J]. Lung Cancer, 2023, 175: 68−78. doi: 10.1016/j.lungcan.2022.11.016.
[101]
HUANG J, ZHANG X H, CAI Y, et al. Rationale and design of a phase II trial of combined serplulimab and chemotherapy in patients with histologically transformed small cell lung cancer: a prospective, single-arm and multicentre study[J]. Clin Oncol (R Coll Radiol), 2024, 36(1): 39−45. doi: 10.1016/j.clon.2023.11.030.
[102]
LAI L, MENG W T, WEI J L, et al. Transformation of NSCLC to SCLC after 1st- and 3rd-generation EGFR-TKI resistance and response to EP regimen and erlotinib: 2 CARE-compliant case reports[J]. Medicine (Baltimore), 2021, 100(10): e25046. doi: 10.1097/MD.0000000000025046.
[103]
YANG H Y, LIU L, ZHOU C H, et al. The clinicopathologic of pulmonary adenocarcinoma transformation to small cell lung cancer[J]. Medicine (Baltimore), 2019, 98(12): e14893. doi: 10.1097/md.0000000000014893.
[104]
NIEDERST M J, SEQUIST L V, POIRIER J T, et al. RB loss in resistant EGFR mutant lung adenocarcinomas that transform to small-cell lung cancer[J]. Nat Commun, 2015, 6: 6377. doi: 10.1038/ncomms7377.
[105]
PAPAVASSILIOU K A, SOFIANIDI A A, GOGOU V A, et al. P53 and Rb aberrations in small cell lung cancer (SCLC): from molecular mechanisms to therapeutic modulation[J]. Int J Mol Sci, 2024, 25(5): 2479. doi: 10.3390/ijms25052479.
[106]
GONG X Q, DU J, PARSONS S H, et al. Aurora A kinase inhibition is synthetic lethal with loss of the RB1 tumor suppressor gene[J]. Cancer Discov, 2019, 9(2): 248−263. doi: 10.1158/2159-8290.CD-18-0469.
[107]
SHAH K N, BHATT R, ROTOW J, et al. Aurora kinase A drives the evolution of resistance to third-generation EGFR inhibitors in lung cancer[J]. Nat Med, 2019, 25(1): 111−118. doi: 10.1038/s41591-018-0264-7.
[108]
SHAUROVA T, ZHANG L T, GOODRICH D W, et al. Understanding lineage plasticity as a path to targeted therapy failure in EGFR-mutant non-small cell lung cancer[J]. Front Genet, 2020, 11: 281. doi: 10.3389/fgene.2020.00281.
[109]
GALLARDO A, MOLINA A, ASENJO H G, et al. EZH2 endorses cell plasticity to non-small cell lung cancer cells facilitating mesenchymal to epithelial transition and tumour colonization[J]. Oncogene, 2022, 41(28): 3611−3624. doi: 10.1038/s41388-022-02375-x.
[110]
DUAN R, DU W F, GUO W J. EZH2: a novel target for cancer treatment[J]. J Hematol Oncol, 2020, 13(1): 104. doi: 10.1186/s13045-020-00937-8.
全文刊登于《循证医学》2026年 第26卷 第1期
↓↓↓ 点击"阅读原文" 获取详细全文信息
声明:本文由“肿瘤界”整理与汇编,欢迎分享转载,如需使用本文内容,请务必注明出处。
来源:循证医学杂志
编辑:Lagertha
审核:素问
临床结果抗体药物偶联物申请上市临床研究
2026-03-03
·美通社
纽约
2026年3月3日
/美通社/ -- 生物制药特许权买家Royalty Pharma plc(纳斯达克代码:RPRX)宣布任命孙锶铭先生 (Kenneth Sun)为高级副总裁兼亚洲区主管,自2026年5月起生效。他将常驻香港,领导公司在亚洲的特许权业务。在加入Royalty Pharma 前,他担任摩根士丹利亚太区医疗投资银行部主管。
亚洲生物科技企业如今积极研发了多种创新治疗药物。在2025年,光是中国创新药物的对外授权,已公告的交易额就超过1,300亿美元,远高于2021年的约140亿美元,反映跨国制药企业越来越重视这类创新成果。随着亚洲在分子类型、治疗领域及交易结构日益创新、全面且多元化,这一趋势预计将延续至2026年及未来很长时间。
这些交易所带来的特许权使用费,正在推动以特许权为基础的融资市场新机遇。Royalty Pharma将与创新生物科技企业携手在亚洲各地开拓特许权市场,正如二十多年前Royalty Pharma与大学、研究机构及生物制药公司紧密合作,奠定了西方生物制药特许权市场的基础。
Royalty Pharma首席执行官兼董事会主席Pablo Legorreta表示:"我们非常欢迎Ken的加入,他对于亚洲蓬勃发展中的创新所带来的融资需求,有深刻而具战略性的理解。要深耕亚洲市场,具备当地专业知识和强大的人脉关系至关重要。我们认为,透过特许权及其他新型融资方式支持创新,在亚洲拥有极具潜力的长期发展机遇。Ken 将发挥关键作用,助力我们进一步扩大全球业务平台。"
孙锶铭先生表示:"我非常荣幸能加入Royalty Pharma,这家特许权市场的先驱和无可争议的领导企业。亚洲特许权市场发展迅速,为创新生物科技公司带来了具有规模、灵活且无需稀释股权的全新资金来源。现时正值亚洲生物制药创新加速发展之际,我非常期待推动特许权市场的建立与成长。"
在加入Royalty Pharma之前,孙锶铭先生曾任摩根士丹利董事总经理兼亚太区医疗投资银行部主管,带领该行在中国建立市场领导地位。他曾为不同类型的战略客户提供投资银行服务,包括生物科技、医疗器械、医药研发外包服务机构(CRO)、制药及医疗服务公司,并专注于大中华区。他曾成功领导和完成多宗具有里程碑意义和行业标竿性的医疗行业交易,包括首次公开发行、企业并购及生物科技企业对外授权交易。他有超过18年的投资银行经验,曾于华兴资本及法国巴黎银行任职。他毕业于加拿大皇后大学,拥有电机工程学士学位。
关于
Royalty Pharma
Royalty Pharma 成立于 1996 年,是生物制药特许权的最大买家,也是生物制药产业创新的领先资助机构。Royalty Pharma 与来自学术机构、研究医院、非营利组织、中小型生物科技公司及全球领先制药企业的创新者合作。Royalty Pharma 的特许权组合使其有权根据多项业界领先疗法的销售额直接获得收益。目前,其特许权组合涵盖 35 种以上的商业化产品,包括 Vertex 的 Trikafta 和 Alyftrek、GSK 的 Trelegy、Biogen 的 Tysabri 和 Spinraza、Roche 的 Evrysdi、Astellas 和 Pfizer 的 Xtandi、Johnson & Johnson 的 Tremfya、AbbVie 和 Johnson & Johnson 的 Imbruvica、Servier 的 Voranigo、Gilead 的 Trodelvy、Amgen 的 Imdelltra 及 Alnylam 的 Amvuttra 等,以及 20 项处于研发阶段的候选产品。欲了解更多信息,请访问
www.royaltypharma.com
。
前瞻性声明
本文所载资讯并非旨在完整呈现或涵盖您可能需要的所有资讯。除非另有说明,本文所载声明均以本文件日期为准。无论何时交付本文件或进行任何证券销售行为,均不得在任何情况下暗示本文所载资讯于该日期之后仍然正确,亦不得暗示该资讯将更新或修订以反映后续取得的资讯或本文日期后发生的变更。
本文内容包含根据美国 1995 年《私人证券诉讼改革法案》定义的“前瞻性声明”,包括表达公司对未来事件或结果之预测的观点、预期、信念、计划、目标、假设或预测,有别于反映历史事实的陈述。例子包括关于 Royalty Pharma的 策略、融资计划、增长机会、市场增长及资本运用计划的讨论。在某些情况下,您可以通过“预期”、“打算”、”相信”、“估计”、“计划”、“寻求”、“预计”、“期望”、“可能”、“将”、“会”、“可以”或“应该”等词语及其否定形式或类似表述来识别这些前瞻性声明。前瞻性声明基于管理层目前的信念、假设及目前获得的资讯。然而,这些前瞻性声明无法保证Royalty Pharma 的业绩表现,您不应过度依赖这些声明。前瞻性声明受到多项风险、不确定性及其他变数的影响,这些因素可能导致声明不准确,再次提醒读者不应过度依赖此类声明。许多风险超出公司控制范围,可能导致实际结果与预期有重大差异。本文件所含前瞻性声明仅以本文件日期为准。除法律要求外,公司不承担亦明确拒绝任何义务更新此类声明或公开宣布任何修正结果,以反映未来事件或发展。
本文所载部分资讯涉及或基于第三方来源的研究、出版物、调查及其他数据,以及公司内部估算和研究。尽管公司认为此类第三方来源截至本文件日期具可靠性,但并未独立核实,也不对任何来自第三方来源的资讯之充分性、公平性、准确性或完整性作出任何声明。此外,本文所载的市场数据均涉及若干假设及限制,无法保证此类假设之准确性或可靠性。最后,尽管公司相信其内部研究具可靠性,但该等研究未经任何独立来源验证。
有关更多资讯,请参阅 Royalty Pharma 向美国证券交易委员会(SEC)提交的报告及文件,可透过 SEC 官网的 EDGAR 系统查阅:
www.sec.gov
。
Royalty Pharma
投资者关系与传讯部
+1 (212) 883-6772
ir@royaltypharma.com
2026-03-02
·多盟
2025年末,细胞治疗领域正经历一场“大退潮”。武田(Takeda)宣布全面退出,诺和诺德(Novo Nordisk)终止所有T细胞治疗项目.
T细胞疗法接近身体自然环境、副作用小、治愈率高, 但这两个巨头放弃, 什么原因?
真实的原因并不是因为细胞疗法“没用”或“治愈率不高”, 而是一些大药企算账后觉得短期内回报不成比例。大药企的逻辑是: 如果一个疗法能“一次性治断根”,患者就不再需要终身服药或反复治疗,那些每年贡献数百亿的“现金奶牛”药物(如GLP-1类、某些慢性病生物制剂)就会失去长期收入来源。
所以, 大药企更偏好“终身管理”的慢性疗法,让患者“治而不死”, 因为这种情况下病人们就成了可提供稳定、可预测现金的现金奶牛,而对一次性治愈方式, 则会破坏这个模式——这是资本主义逐利逻辑下的自然结果。
治标不治本的公司及药物代表如下:
GLP-1 类
代表药物为司美格鲁肽与替尔泊肽, 只是模拟肠道激素,控制血糖/食欲/体重,但停药后体重/血糖反弹率高(80-90%),不修复胰岛β细胞功能。基本只是治标, 压根儿不治本。
ADC类
代表药物为Enhertu、Trodelvy、Padcev、Datopotamab deruxtecan 等,主要应用于治疗乳腺癌、肺癌、尿路上皮癌、胃癌等实体瘤。多数治标,只有少数接近治本。它们可以精准递送毒素去杀癌细胞,但肿瘤异质性/抗原丢失会导致耐药;晚期/转移瘤多为延长生存而非根除肿瘤。其比传统化疗“治本”潜力大,但远不如CAR-T在血液瘤的持久CR率。
口服小分子类
代表药物为Imatinib (慢粒)、Osimertinib (EGFR肺癌)、Larotrectinib (NTRK融合)。针对驱动基因阻断信号通路;慢粒用TKI可实现“功能性治愈”(停药后多数不复发);但实体瘤多产生耐药性。而且实体瘤多需长期服药。部分真正治本,多数只是治标。
双抗 (BsAb)
代表有Blinatumomab、Teclistamab、Epcoritamab、Mosunetuzumab 等, 应用于多发性骨髓瘤、淋巴瘤、部分实体瘤,但多数只治标,只有少数接近治本
细胞疗法与基因编辑疗法, 原理上最接近治本,部分已实现,部分患者10年+无复发;代表包括CAR-T (Kymriah等)、CAR-NK、异体NK(如ImmunityBio),Prime基因编辑(Prime Medicine), 一次/有限次输注,产生免疫记忆;某些B细胞瘤CR率40-60%,长期无病存活(功能性治愈)部分患者10年+无复发;细胞疗法及基因编辑疗法治愈疾病及癌症的潜力最高,但规模化仍存瓶颈(个性化制造慢、成本高、物流复杂、质量一致性差)。为了克服规模化个性化问题, 领先的细胞及基因编辑公司正通过四大核心策略快速突破,目标是将“贵族疗法”变成“生物类似药级”可及产品(成本降至1-2万美元/剂、年产数万剂、门诊即用)。
1. 异体现成(off-the-shelf)+ 基因编辑消除排斥(最直接的规模化路径)
从“患者自细胞”转向“健康捐献者或工程细胞库”,一次生产可供多人使用。
-ImmunityBio:已完成64名癌症患者+健康捐献者的M-ceNK(记忆型细胞因子增强NK细胞)制造工艺开发,所有细胞冷冻储存,形成“自然杀手细胞世界银行”。这些细胞专为机器人/AI制造训练,结合Anktiva(IL-15)实现广谱抗癌。临床上,QUILT-3.076(实体瘤Phase 1)和ResQ209(卵巢癌Phase 2)已推进,无需患者个性化制造。
-Allogene Therapeutics:2026年定位为“程序定义年”,目标实现“生物类似药规模”(年产3-6万剂、成本<1-2万美元/剂)。其AlloCAR-T平台已治疗200+患者,Dagger®技术可靶向清除排斥细胞,减少或取消传统淋巴耗竭化疗。Phase 2 ALPHA3(淋巴瘤)和ALLO-329(自身免疫病)2026上半年将有关键数据,目标社区医院门诊使用。
-Fate Therapeutics/Nkarta用iPSC(诱导多能干细胞)母细胞库大规模生产CAR-NK;
-CRISPR Therapeutics用TALEN/CRISPR敲除HLA/TCR基因,避免GvHD。
2. 自动化、机器人+AI制造(解决人工瓶颈)
传统制造依赖手工,失败率高、周期长。现在转向全封闭、自动化系统。
- ImmunityBio的Leonardo AI机器人已用64批次细胞完成训练,目标未来全AI驱动生产,极大降低成本并实现“世界银行”库存规模化.
-Cellular Origins获4000万美元融资开发自动化机器人平台;Thermo Fisher、Lonza等CDMO推出“GMP-in-a-box”一体机,制造时间从几周缩短至几天,批次失败率大幅下降。2026年行业预测:自动化+数字孪生将使COGS(制造成本)降50%以上。
3. 体内(in vivo)基因编辑——革命性跳过体外制造最突破性的解决方案
体内(in vivo)基因编辑不再提取细胞,而是用脂质纳米颗粒(LNP)直接把CRISPR编辑器注射进体内(主要靶向肝脏或造血干细胞),一次输注即可完成编辑。
-CRISPR Therapeutics:LNP平台推进CTX310(ANGPTL3,心血管)和CTX320(Lp(a))肝脏编辑,单次静脉注射即实现持久降脂。SyNTase™新一代编辑技术更精准可规模化,2026年将有多个更新,并启动AATD(α-1抗胰蛋白酶缺乏症)和高血压项目。优势:无需患者特异性制造,可覆盖数十万患者。
-Intellia Therapeutics:in vivo CRISPR领先者,ATTR(转甲状腺素淀粉样变)和HAE(遗传性血管水肿)Phase 3已完成入组,2026年上半年有望出顶线数据,BLA提交在即。单次输注即可实现93%蛋白敲低,彻底绕过细胞疗法的制造/物流难题。
4. iPSC干细胞库 + 闭合系统 + 去中心化生产
用iPSC建立“永生”细胞库,无限扩增一致性细胞(Fate、Century Therapeutics)。
闭合自动化系统(减少人为污染)和点-of-care(医院现场制造)结合,解决冷链物流问题。
行业整体上看, 2026年CDMO和自动化投资激增,目标是让细胞/基因疗法从“定制奢侈品”变成常规治疗。
这些勇敢创新的公司没有“坐等”大药企,也不畏大药企的资金优势, 而是坚持“根治”信仰, 通过异体+自动化+体内编辑的三管齐下,把规模化瓶颈逐一击破。
2026年将是“从概念到现实”的转折年——一旦关键读数正面,患者治疗成本将可降至可报销水平,治愈性疗法将真正从“少数人专享”走向“大多数患者可及”。当然,实体瘤完全治愈仍需时间,但方向已明确。
100 项与 戈沙妥组单抗 相关的药物交易
登录后查看更多信息
研发状态
批准上市
10 条最早获批的记录, 后查看更多信息
登录
| 适应症 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|
| 转移性三阴性乳腺癌 | 欧盟 | 2021-11-22 | |
| 转移性三阴性乳腺癌 | 冰岛 | 2021-11-22 | |
| 转移性三阴性乳腺癌 | 列支敦士登 | 2021-11-22 | |
| 转移性三阴性乳腺癌 | 挪威 | 2021-11-22 | |
| 乳腺癌 | 瑞士 | 2021-09-09 | |
| HR阳性/HER2阴性乳腺癌 | 澳大利亚 | 2021-09-06 | |
| 尿路上皮癌 | 美国 | 2021-04-13 | |
| 三阴性乳腺癌 | 美国 | 2020-04-22 |
未上市
10 条进展最快的记录, 后查看更多信息
登录
| 适应症 | 最高研发状态 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|---|
| 转移性乳腺癌 | 申请上市 | 中国 | 2021-05-17 | |
| 广泛期小细胞肺癌 | 临床3期 | 美国 | 2025-04-04 | |
| 广泛期小细胞肺癌 | 临床3期 | 中国 | 2025-04-04 | |
| 广泛期小细胞肺癌 | 临床3期 | 日本 | 2025-04-04 | |
| 广泛期小细胞肺癌 | 临床3期 | 阿根廷 | 2025-04-04 | |
| 广泛期小细胞肺癌 | 临床3期 | 澳大利亚 | 2025-04-04 | |
| 广泛期小细胞肺癌 | 临床3期 | 比利时 | 2025-04-04 | |
| 广泛期小细胞肺癌 | 临床3期 | 巴西 | 2025-04-04 | |
| 广泛期小细胞肺癌 | 临床3期 | 加拿大 | 2025-04-04 | |
| 广泛期小细胞肺癌 | 临床3期 | 法国 | 2025-04-04 |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
临床2期 | 肌层浸润性膀胱癌 辅助 | 新辅助 | 49 | Sacituzumab govitecan (SG) plus Pembrolizumab (Pembro) (cCR) | 簾鹹衊選糧遞憲鹹獵艱(觸襯範選鏇簾衊襯獵遞) = 艱顧齋築獵構鬱壓廠夢 遞艱遞獵獵範壓鏇壓獵 (構廠鬱淵觸願夢憲蓋膚 ) 更多 | 积极 | 2026-02-26 | |
Sacituzumab govitecan (SG) plus Pembrolizumab (Pembro) (non-cCR) | 簾鹹衊選糧遞憲鹹獵艱(觸襯範選鏇簾衊襯獵遞) = 襯廠鹹窪醖簾構簾鏇憲 遞艱遞獵獵範壓鏇壓獵 (構廠鬱淵觸願夢憲蓋膚 ) 更多 | ||||||
临床2期 | 223 | (Cohort 1 Group 2: NSCLC (Adenocarcinoma)) | 觸繭淵膚衊範獵襯餘顧 = 築壓鹹糧觸鹽齋鹹餘糧 選窪築願鏇糧衊構遞築 (艱蓋鹹觸餘衊選齋選膚, 糧廠築壓淵夢顧積鬱膚 ~ 範繭簾遞醖淵鏇獵壓簾) 更多 | - | 2026-01-30 | ||
(Cohort 1 Group 3: NSCLC (SCC)) | 觸繭淵膚衊範獵襯餘顧 = 餘願夢膚壓觸鑰構範簾 選窪築願鏇糧衊構遞築 (艱蓋鹹觸餘衊選齋選膚, 廠製憲蓋壓構願範鏇構 ~ 製製選齋蓋餘鹽衊淵糧) 更多 | ||||||
临床3期 | 晚期三阴性乳腺癌 PD-L1-positive | 443 | 鬱齋簾襯獵獵築製糧網(蓋鹽廠窪構網淵網鑰齋) = 顧襯築選衊構獵憲鹽膚 淵憲糧鹽築簾餘鬱範壓 (鹽壓憲遞窪鏇獵壓構廠, 12.7 ~ 19.5) 更多 | 积极 | 2026-01-22 | ||
Chemotherapy plus pembrolizumab | 鬱齋簾襯獵獵築製糧網(蓋鹽廠窪構網淵網鑰齋) = 憲構糧窪膚淵願鹽鹹鏇 淵憲糧鹽築簾餘鬱範壓 (鹽壓憲遞窪鏇獵壓構廠, 7.6 ~ 11.3) 更多 | ||||||
临床2期 | 2 | Sacituzumab Govitecan (SG) (Arm 1/Cohort 3 -Treatment With Sacituzumab Govitecan (SG)) | 鹽觸範鏇繭選網獵願淵 = 鑰糧簾齋膚壓憲選鏇鑰 鹽襯選積淵製齋範製壓 (構範醖壓糧鹹築淵遞醖, 選壓蓋鬱憲餘選鏇選鏇 ~ 鏇夢衊網願鹽鹽願壓憲) 更多 | - | 2026-01-21 | ||
Sacituzumab (Participants Not Assigned to an Arm/Cohort - Treatment With Sacituzumab Govitecan (SG)) | 鹽觸範鏇繭選網獵願淵 = 夢壓壓鏇齋築艱構選鑰 鹽襯選積淵製齋範製壓 (構範醖壓糧鹹築淵遞醖, 築鬱遞蓋膚鹽積蓋遞製 ~ 鑰夢壓顧夢艱獵憲鑰獵) 更多 | ||||||
临床2期 | 30 | 觸憲觸積選簾獵積觸範(願壓顧夢製製蓋壓艱艱) = 網醖糧獵襯範鹽獵糧壓 獵鏇鹹鏇憲積構範鑰範 (壓衊襯簾蓋憲願選繭壓 ) | 积极 | 2025-12-12 | |||
N/A | 三阴性乳腺癌 二线 | 42 | 齋觸繭衊鏇齋積積醖窪(蓋簾願築壓糧壓艱鏇觸) = Grade 2 diarrhea occurred in 7.1%, grade 1 in 16.7%. Grade 3 and 4 neutropenia were observed in 4.8% each. Granulocyte colony-stimulating factors use was reported in 23.8% of patients. 遞構構淵願憲鬱夢願夢 (壓淵鑰憲膚網壓繭醖艱 ) | 积极 | 2025-12-12 | ||
N/A | 303 | (>65 years) | 蓋鏇齋鑰範願鹽壓餘觸(築壓憲築艱鹽遞膚鏇觸) = 夢憲製繭顧餘網鹽醖膚 鏇夢窪衊衊觸鏇糧鑰製 (構廠鏇蓋壓鬱鏇觸範壓 ) 更多 | 积极 | 2025-12-12 | ||
(≤65 years) | 蓋鏇齋鑰範願鹽壓餘觸(築壓憲築艱鹽遞膚鏇觸) = 蓋憲願窪鹹膚襯積膚衊 鏇夢窪衊衊觸鏇糧鑰製 (構廠鏇蓋壓鬱鏇觸範壓 ) 更多 | ||||||
临床2期 | 50 | 製壓艱蓋觸夢蓋獵鹹鬱(繭蓋憲餘築鹽範蓋夢膚) = 構壓願膚夢鬱壓網淵顧 網鹹憲齋壓鹹壓蓋鑰醖 (蓋廠構製膚觸鬱簾襯鬱, 0.99 ~ 7.65) 更多 | 积极 | 2025-12-11 | |||
N/A | 晚期乳腺癌 ER positive | HER2 negative | 74 | 顧築齋鑰鏇製襯製鏇齋(齋網醖醖襯膚積衊窪遞) = 鏇鏇簾製憲獵窪鑰壓網 鏇淵簾願觸鹹選鏇鹹艱 (壓遞鏇積艱繭範衊鹹鹹, 9 ~ 20) 更多 | 不佳 | 2025-12-10 | ||
N/A | HR阳性/HER2阴性乳腺癌 HER2-low | HR+ | HER2-0 | 472 | (HR+/HER2- mBC) | 淵鏇範範築齋衊廠鏇淵(鬱艱網鏇繭選壓築鹹鏇) = 醖鬱衊鹽餘繭獵鏇壓蓋 憲蓋壓範醖憲憲選鏇廠 (餘鑰獵鬱齋蓋鑰鏇簾糧, 10.2 ~ not estimable) 更多 | 积极 | 2025-12-10 | |
(mTNBC) | 淵鏇範範築齋衊廠鏇淵(鬱艱網鏇繭選壓築鹹鏇) = 廠憲築夢構憲淵積範選 憲蓋壓範醖憲憲選鏇廠 (餘鑰獵鬱齋蓋鑰鏇簾糧, 13.6 ~ 20.3) 更多 |
登录后查看更多信息
转化医学
使用我们的转化医学数据加速您的研究。
登录
或

药物交易
使用我们的药物交易数据加速您的研究。
登录
或

核心专利
使用我们的核心专利数据促进您的研究。
登录
或

临床分析
紧跟全球注册中心的最新临床试验。
登录
或

批准
利用最新的监管批准信息加速您的研究。
登录
或

生物类似药
生物类似药在不同国家/地区的竞争态势。请注意临床1/2期并入临床2期,临床2/3期并入临床3期
登录
或

特殊审评
只需点击几下即可了解关键药物信息。
登录
或

生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用







