预约演示
更新于:2025-09-09

Celon Pharma SA
更新于:2025-09-09
概览
标签
肿瘤
神经系统疾病
呼吸系统疾病
小分子化药
mRNA疫苗
mRNA
疾病领域得分
一眼洞穿机构专注的疾病领域
暂无数据
技术平台
公司药物应用最多的技术
暂无数据
靶点
公司最常开发的靶点
暂无数据
| 排名前五的药物类型 | 数量 |
|---|---|
| 小分子化药 | 10 |
| mRNA疫苗 | 3 |
| mRNA | 2 |
| 重组蛋白 | 2 |
| 治疗性疫苗 | 2 |
关联
19
项与 Celon Pharma SA 相关的药物作用机制 GR激动剂 [+2] |
在研机构 |
原研机构 |
最高研发阶段批准上市 |
首次获批国家/地区 美国 |
首次获批日期2000-08-24 |
靶点 |
作用机制 GPR40激动剂 |
在研机构 |
原研机构 |
在研适应症 |
非在研适应症 |
最高研发阶段临床2期 |
首次获批国家/地区- |
首次获批日期- |
作用机制 JAK1抑制剂 [+3] |
在研机构 |
原研机构 |
最高研发阶段临床2期 |
首次获批国家/地区- |
首次获批日期- |
18
项与 Celon Pharma SA 相关的临床试验CTIS2024-514725-38-00
- 01INDA2024
开始日期2025-06-01 |
申办/合作机构 |
CTIS2024-519660-40-00
- 02API2024
开始日期2025-04-06 |
申办/合作机构 |
CTIS2024-511526-31-00
- 01API2024
开始日期2024-09-01 |
申办/合作机构 |
100 项与 Celon Pharma SA 相关的临床结果
登录后查看更多信息
0 项与 Celon Pharma SA 相关的专利(医药)
登录后查看更多信息
47
项与 Celon Pharma SA 相关的文献(医药)2025-06-01·iScience
Mapping protein-metabolite interactions in E. coli by integrating chromatographic techniques and co-fractionation mass spectrometry
Article
作者: Zillmer, Hanne ; Schroeder, Frank C ; Wagner, Mateusz ; Gorka, Michal ; Dehesh, Katayoon ; Walther, Dirk ; Plecki, Caroline F ; Guo, Jingzhe ; Mercado, Catherine ; Kang, Jieun ; Thirumlaikumar, Venkatesh P ; Skirycz, Aleksandra ; Minen, Romina I
Toward characterization of protein-metabolite interactomes, we recently introduced PROMIS, a co-fractionation-based mass spectrometry approach. However, the challenge lies in distinguishing true interactors from coincidental co-elution when a metabolite co-fractionates with numerous proteins. To address this, we integrated two chromatographic techniques-size exclusion and ion exchange-to enhance the mapping of protein-metabolite interactions (PMIs) in Escherichia coli. This integration aims to refine the PMI network by considering size and charge characteristics, resulting in 994 interactions involving 51 metabolites and 465 proteins. The PMI network is enriched for known and predicted interactions, providing validation. Furthermore, analyzing protein targets for different metabolites revealed functional insights, such as a connection between proteinogenic dipeptides and fatty acid biosynthesis. Notably, we uncovered an inhibitory interaction between the riboflavin degradation product lumichrome and orotate phosphoribosyltransferase, a key enzyme in de novo pyrimidine synthesis affecting biofilm formation. In summary, our integrated chromatographic approach significantly advances PMI mapping.
2025-03-24·Journal of Chemical Information and Modeling
From NMR to AI: Do We Need 1H NMR Experimental Spectra to Obtain High-Quality logD Prediction Models?
Article
作者: Świderska, Aleksandra ; Pietruś, Wojciech ; Kurczab, Rafał ; Leniak, Arkadiusz
This study presents a novel approach to 1H NMR-based machine learning (ML) models for predicting logD using computer-generated 1H NMR spectra. Building on our previous work, which integrated experimental 1H NMR data, this study addresses key limitations associated with experimental measurements, such as sample stability, solvent variability, and extensive processing, by replacing them with fully computational workflows. Benchmarking across various density functional theory (DFT) functionals and basis sets highlighted their limitations, with DFT-based models showing relatively high RMSE values (average CHI logD of 1.12, lowest at 0.96) and extensive computational demands, limiting their usefulness for large-scale predictions. In contrast, models trained on predicted 1H NMR spectra by NMRshiftDB2 and JEOL JASON achieved RMSE values as low as 0.76, compared to 0.88 for experimental spectra. Further analysis revealed that mixing experimental and predicted spectra did not enhance accuracy, underscoring the advantage of homogeneous datasets. Validation with external datasets confirmed the robustness of our models, showing comparable performance to commercial software like Instant JChem, thus underscoring the reliability of the proposed computational workflow. Additionally, using normalized RMSE (NRMSE) proved essential for consistent model evaluation across datasets with varying data scales. By eliminating the need for experimental input, this workflow offers a widely accessible, computationally efficient pipeline, setting a new standard for ML-driven chemical property predictions without experimental data constraints.
2025-03-04·DRUG DEVELOPMENT AND INDUSTRIAL PHARMACY
Formulation development and scale-up of dutasteride liquisolid tablets
Article
作者: Mendyk, Aleksander ; Antosik-Rogóż, Agata ; Huszcza, Grzegorz ; Jachowicz, Renata ; Pesta, Edyta ; Woyna-Orlewicz, Krzysztof ; Kurek, Mateusz ; Dorożyński, Przemysław ; Strózik, Mirosław
INTRODUCTION:
Liquisolid (LS) technology is particularly advantageous for poorly water-soluble drugs administered in very low doses because of the improved dissolution rate and superior content uniformity. However, there is a lack of research papers describing the application of this concept on an industrial scale. Thus, we present trials conducted to develop tablets containing 0.5 mg of water-insoluble dutasteride (DTS) according to the LS approach.
METHODS:
We divided the study into two stages: developing a placebo formulation and producing LS tablets containing DTS on a pilot scale. We tested all the manufactured tablets for mass uniformity, resistance to crushing, disintegration time, dissolution, stability, and presence of impurities.
RESULTS:
We demonstrated that a standard high-shear granulator mixer with a spraying system is effective for LS formulation development and transfer to the pilot scale. We were able to compress the system into tablets with the desired assay, content uniformity, dissolution, and mechanical strength.
CONCLUSION:
Multiple operations can be performed on one piece of equipment - that is, pre-mixing a carrier, wetting of the carrier with a solution of an active ingredient in a nonvolatile liquid, mixing of the resulted mass with a coating agent, as well as additional excipients. Preparation of powder blends ready for tableting in line with the one-pot process approach is especially advantageous for the safety of staff engaged in the manufacturing of highly potent drug products.
28
项与 Celon Pharma SA 相关的新闻(医药)2025-09-03
The launch of emerging therapies like BESREMi (PharmaEssentia and AOP Orphan Pharmaceuticals), INCB057643 (Incyte), XPOVIO (Karyopharm Therapeutics), RYTELO (Geron), REBLOZYL (Bristol Myers Squibb), Navtemadlin (Kartos Therapeutics), Pelabresib (Novartis), and others is going to shift the myelofibrosis market.
LAS VEGAS, Sept. 3, 2025 /PRNewswire/ -- DelveInsight's
Myelofibrosis Market Insights report includes a comprehensive understanding of current treatment practices, emerging myelofibrosis drugs, market share of individual therapies, and current and forecasted myelofibrosis market size from 2020 to 2034, segmented into leading markets (the US, EU4, UK, and Japan).
Myelofibrosis Market Summary
The total myelofibrosis treatment market size in the leading markets (the US, EU4, UK, and Japan) was
USD 2.2 billion in 2024.
The United States accounts for the largest market size of myelofibrosis, in comparison to EU4 (Germany, Italy, France, and Spain) and the UK, and Japan.
Based on DelveInsight's assessment in 2024, the 7MM had approximately
56K patient pool (prevalent cases) of myelofibrosis.
Key myelofibrosis companies, including
PharmaEssentia, AOP Orphan Pharmaceuticals, Incyte, Karyopharm Therapeutics, Geron, Bristol Myers Squibb, Kartos Therapeutics, Novartis, Merck, Telios Pharma, Ryvu Therapeutics, Sumitomo Pharma, Syntara, Disc Medicine, Menarini Group, and others, are actively working on innovative myelofibrosis drugs.
Some of the key myelofibrosis therapies in clinical trials include
BESREMi (ropeginterferon alfa-2b-njft/P-1101), INCB057643, XPOVIO (NEXPOVIO/selinexor/KPT-330), RYTELO (imetelstat), REBLOZYL (luspatercept/ACE-536), Navtemadlin (KRT-232), Pelabresib (DAK539), Bomedemstat (IMG-7289/MK-3543), TL-895, RVU120 ( SEL-120), TP-3654 (nuvisertib), SNT-5505 (PXS-5505), DISC-0974, ELZONRIS (tagraxofusp/SL-401), and others. These novel myelofibrosis therapies are anticipated to enter the myelofibrosis market in the forecast period and are expected to change the market.
By 2034, among all the therapies, the highest revenue is expected to be generated by
OJJAARA/OMJJARA.
Discover which myelofibrosis therapies are expected to grab the largest market share @
Myelofibrosis Market Report
Key Factors Driving the Growth of the Myelofibrosis Market
Novel Therapies Drive Myelofibrosis Treatment Advancements
Currently, four JAK inhibitors have been approved by the US FDA for the treatment of myelofibrosis, including JAKAFI (ruxolitinib), INREBIC (fedratinib), VONJO (pacritinib), and OJJAARA (momelotinib). No drug therapy can cure myelofibrosis. As myelofibrosis primarily affects older adults, stem cell transplantation is not a treatment option for most myelofibrosis patients. The launch of another JAK inhibitor, BESREMi, will further change the dynamics of the myelofibrosis market.
Rich clinical pipeline and emerging non-JAK approaches driving the myelofibrosis landscape
Beyond JAK inhibitors, active clinical development includes
BET inhibitors (Incyte's INCB057643; Novartis' Pelabresib),
XPO1 inhibitor (Karyopharm Therapeutics' XPOVIO),
Telomerase inhibitor (Geron's RYTELO),
MDM2 protein inhibitor (Kartos Therapeutics' Navtemadlin),
Tyrosine kinase inhibitors (Telios Pharma's TL-895),
PIM1 kinase inhibitor (Sumitomo Pharma's TP-3654),
LOX inhibitor (Syntara's SNT-5505), and others, raising market potential by promising new label expansions, second-line options, and higher-value therapies should any prove to be disease-modifying.
An aging population & increasing incidence with better survival
Myelofibrosis primarily affects older adults; population aging increases the absolute number of patients. As treatments improve symptoms and (in some cases) survival, prevalent patient pools grow, supporting longer-term market demand. In the US in 2024, the 70+ years of age group had the highest number of cases, accounting for approximately
60% of the total prevalent cases, while the ≤39 years of age group accounted for just
~2%.
Diagnostic & genomic testing improvements are driving myelofibrosis patient pool, leading to the growth of myelofibrosis market
Wider use of molecular profiling (JAK2, CALR, MPL, and others) enables earlier and more accurate MF diagnosis and better patient stratification for targeted therapies. This both expands the diagnosed population and helps match patients to appropriate, often higher-value, treatments.
Myelofibrosis Market Analysis
JAK inhibitors have become the cornerstone of treatment for patients with myelofibrosis, offering significant benefits such as spleen reduction, symptom relief, and improved quality of life, which can also extend survival in those with advanced disease. All approved JAK inhibitors primarily target JAK2, particularly the wild-type form, but they differ in their activity against other JAK family members.
For instance, JAKAFI myelofibrosis inhibits both JAK1 and JAK2; INREBIC selectively inhibits JAK2 while sparing JAK1 and also affects FLT3 and other targets; VONJO myelofibrosis inhibits JAK2 while sparing JAK1 but additionally impacts FLT3, IRAK, and ACVR1; and OJJAARA, approved through a different pathway, targets JAK1/JAK2 and ACVR1, mainly for myelofibrosis patients with anemia. These varying mechanisms lead to distinct patient outcomes.
JAKAFI myelofibrosis continues to see strong demand and is expected to grow further, maintaining its position as the standard of care in myelofibrosis. Myelofibrosis will remain the largest segment of JAKAFI's patient population until polycythemia vera cases eventually increase. However, market growth may be limited due to patent expirations of key therapies, with JAKAFI patents set to expire in 2027 for Novartis and 2028 for Incyte, presenting potential opportunities for competitors. In response, Incyte is exploring combination trials with novel drugs to extend JAKAFI's therapeutic lifespan.
Learn more about the treatment options for myelofibrosis @
Myelofibrosis Therapy
The myelofibrosis treatment landscape offers significant opportunities for innovation, focusing on several key areas:
Treating Lower-Risk Patients: Developing safe and effective therapies for patients with lower-risk myelofibrosis, allowing for earlier and more favorable interventions.
Enhancing First-Line Treatments: Improving existing first-line therapies for intermediate- to high-risk patients through novel drugs or combination strategies.
Managing Cytopenia: Developing targeted therapies to address cytopenia, a significant unmet need in patient care.
Myelofibrosis Competitive Landscape
The myelofibrosis clinical trial pipeline includes several drugs in mid- and late-stage development that are expected to enter the market during the forecast period. The emerging landscape offers a diverse range of therapeutic alternatives for treatment, including
XPOVIO (Karyopharm Therapeutics),
Elitracet (Takeda/Keros Therapeutics),
Navtemadlin (Kartos Therapeutics),
Pelabresib (Novartis),
INCB057643 (Incyte),
Bomedemstat (Merck), and others, all of which are used in various lines of treatment. The expected launch of these therapies is expected to have a further positive impact on the market.
INCB57643 is an orally administered small molecule. Bromodomain and extra-terminal (BET) proteins act as epigenetic readers that control the expression of key oncoproteins implicated in the development of myelofibrosis and other hematologic malignancies, including B-lymphoma-2, nuclear factor kappa, and c-Myc. In a prior Phase I/II clinical trial, the oral BET inhibitor INCB057643, tested both as a monotherapy and in combination with ruxolitinib, demonstrated favorable tolerability and promising clinical activity in patients with advanced cancers. In its Q2 2025 financial report, the company announced that the combination of INCB057643 with ruxolitinib and INCB57643 (a JAK1/JAK2 and BET inhibitor) is currently being evaluated in a Phase II trial for myelofibrosis.
RYTELO (imetelstat) is an investigational telomerase inhibitor that targets telomerase, selectively eliminating malignant stem and progenitor cells in the bone marrow, which drive diseases like myelodysplastic syndromes (MDS) and myelofibrosis. By blocking the proliferation of these malignant cells, imetelstat supports the recovery of healthy bone marrow and blood cell production and has shown disease-modifying effects and clinical benefits in Phase III trials for myelofibrosis. This mechanism sets imetelstat apart from other approved or investigational therapies for these blood cancers. In January 2025, Geron reported achieving 75% enrollment in the Phase III IMpactMF trial, which is comparing imetelstat to Best Available Therapy (BAT) in intermediate-2 or high-risk myelofibrosis patients who have relapsed or are refractory to JAK inhibitor treatment.
Elritercept is a late-stage investigational activin inhibitor aimed at treating anemia associated with hematologic malignancies, including MDS and myelofibrosis. It is currently undergoing Phase II evaluation in patients with myelofibrosis. In December 2024, Keros Therapeutics presented updated data from this ongoing Phase II trial at ASH 2024. Additionally, in December 2024, Takeda announced an exclusive licensing agreement with Keros to further develop, manufacture, and commercialize elritercept globally, excluding mainland China, Hong Kong, and Macau.
The anticipated launch of these emerging myelofibrosis therapies are poised to transform the myelofibrosis market landscape in the coming years. As these cutting-edge myelofibrosis therapies continue to mature and gain regulatory approval, they are expected to reshape the myelofibrosis market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
To know more about new myelofibrosis treatment, visit @
Myelofibrosis Treatment Market
Recent Developments in the Myelofibrosis Market
In
July 2025, Incyte announced that the Phase I data in patients with myelofibrosis as monotherapy and in combination with ruxolitinib are anticipated in the second half of 2025.
In
June 2025, QIAGEN and Incyte announced a new global collaboration to develop a novel diagnostic panel to support Incyte's extensive portfolio of investigational therapies for patients with myeloproliferative neoplasms (MPNs), including Incyte's monoclonal antibody INCA033989.
In
January 2025, Karyopharm Therapeutics stated that it expects to report top-line results from the Phase III SENTRY trial in the second half of 2025, which could represent a potentially transformative opportunity to establish a new treatment paradigm in myelofibrosis.
What is
Myelofibrosis?
Myelofibrosis is a rare blood cancer marked by the accumulation of scar tissue, or 'fibrosis', in the bone marrow. This excess scar tissue prevents the bone marrow from producing enough healthy blood cells. It belongs to a group of blood cancers called 'myeloproliferative neoplasms (MPNs)', in which the blood cells produced by the bone marrow grow and function abnormally. When myelofibrosis arises independently, without being caused by another bone marrow disorder, it is referred to as primary myelofibrosis. In other cases, it can develop from another MPN, such as polycythemia vera or essential thrombocythemia. When this occurs, it is called secondary myelofibrosis, sometimes specifically termed post-polycythemia vera myelofibrosis or post-essential thrombocythemia myelofibrosis.
Myelofibrosis Epidemiology Segmentation
The myelofibrosis epidemiology section provides insights into the historical and current myelofibrosis patient pool and forecasted trends for the leading markets (the US, EU4, UK, and Japan). It helps recognize the causes of current and forecasted patient trends by exploring numerous studies and views of key opinion leaders.
The myelofibrosis market report proffers epidemiological analysis for the study period 2020–2034 in the leading markets (the US, EU4, UK, and Japan) segmented into:
Total Prevalent Cases of Myelofibrosis
Type-specific Cases of Myelofibrosis
Myelofibrosis Cases Based on Risk Stratification
Age-specific Prevalent Cases of Myelofibrosis
Myelofibrosis Cases Based on Molecular Alterations
Download the report to understand which factors are driving myelofibrosis epidemiology trends @
Myelofibrosis Treatment Drugs
Scope of the
Myelofibrosis
Market Report
Myelofibrosis Therapeutic Assessment: Myelofibrosis current marketed and emerging therapies
Myelofibrosis
Market Dynamics: Conjoint Analysis of Emerging Myelofibrosis Drugs
Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
Myelofibrosis Market Unmet Needs, KOL's views, Analyst's views, Myelofibrosis Market Access and Reimbursement
Discover more about myelofibrosis drugs in development @
Myelofibrosis Clinical Trials
Table of Contents
Related Reports
JAK Inhibitors Market
JAK Inhibitors Market Size, Target Population, Competitive Landscape, and Market Forecast – 2034 report delivers an in-depth understanding of the market trends, market drivers, market barriers, and key JAK inhibitors companies, including
Pfizer, AbbVie, Galapagos, Sierra Oncology, Theravance Biopharma, Dizal Pharmaceutical, Aclaris Therapeutics, Celon Pharma, Incyte Corporation, Gilead Sciences, Reistone Biopharma, Jiangsu Hengrui Medicine Co., MaxiNovel Pharmaceuticals, among others.
Myelofibrosis Clinical Trial Analysis
Myelofibrosis Pipeline Insight
– 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key myelofibrosis companies, including
Geron Corporation, Merck, Ryvu Therapeutics SA, Morphic Therapeutic, iOnctura, Pharmaxis, Nippon Shinyaku, Active Biotech, Incyte Corporation, Sumitomo Pharma America, Inc., Cellenkos, Disc Medicine, among others.
Polycythemia Vera Market
Polycythemia Vera Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key polycythemia vera companies, including
Incyte, Novartis, PharmaEssentia, AOP Orphan Pharmaceuticals, Protagonist Therapeutics, Merck (Imago BioSciences), Italfarmaco, Ionis Pharmaceutical, Silence Therapeutics, Perseus Proteomics, AbbVie, Johnson & Johnson Innovative Medicine, Mabwell (Shanghai) Bioscience, Disc Medicine, GluBio Therapeutics, among others.
Multiple Myeloma Market
Multiple Myeloma Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key multiple myeloma companies, including
Sanofi, Karyopharm Therapeutics, AbbVie, Takeda Pharmaceutical, Celgene, Bristol-Myers Squibb, RAPA Therapeutics, Pfizer, Array Biopharma, Cellectar Biosciences, BioLineRx, Celgene, Aduro Biotech, ExCellThera, Janssen Pharmaceutical, Precision BioSciences, Takeda, Glenmark (Ichnos Sciences SA), Poseida Therapeutics, Molecular Partners AG, Chipscreen Biosciences, AbbVie, Genentech (Roche), Janssen Biotech, Nanjing Legend Biotech, Merck Sharp & Dohme Corp., among others.
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve
.
Connect with us on LinkedIn
| Facebook | Twitter
Contact Us
Shruti Thakur
[email protected]
+14699457679
Logo:
SOURCE DelveInsight Business Research, LLP
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
440k+
Newsrooms &
Influencers
9k+
Digital Media
Outlets
270k+
Journalists
Opted In
GET STARTED
临床2期引进/卖出临床1期上市批准临床结果
2025-08-12
·汇聚南药
8月11日,赛隆药业发布了一则引人注目的人事变动公告,公司董事长兼总裁蔡南桂,董事唐霖,董事兼常务副总裁刘达文,董事兼副总裁李剑峰,董事、副总裁、董事会秘书张旭,董事邓拥军,独立董事潘传云,独立董事陈小辛,独立董事李公奋,副总裁王星纷纷递交了辞职报告,这一消息瞬间引发了资本市场和医药行业的关注。
其中,此次辞职涉及的人员中,蔡南桂作为公司的创始人、董事长,在公司的发展历程中有着举足轻重的地位。他申请辞去董事长、董事、总裁、战略决策委员会主任委员职务,不过辞职后仍将在公司担任其他职务。截至公告披露日,蔡南桂持有公司股份 6751.27 万股,同时持有公司股东珠海横琴新区赛隆聚智投资有限公司 47.23% 股权。公司董事会对蔡南桂在任期间为公司战略决策、合规治理以及稳健经营等方面所做出的重大贡献表达了诚挚的敬意与衷心的感谢。
此外,董事唐霖申请辞去董事、战略决策委员会委员、审计委员会委员职务,辞职后将不再担任公司任何职务,她持有公司股份 722.39 万股。董事兼常务副总裁刘达文申请辞去公司董事、常务副总裁以及多个委员会委员职务,辞职后将继续在公司工作,他持有公司股东珠海横琴新区赛普洛投资中心(有限合伙)65.66 万元出资额,以及公司股东珠海横琴新区赛隆聚智投资有限公司 4.09% 股权。董事兼副总裁李剑峰申请辞去公司董事、战略决策委员会委员职务,之后将继续担任公司副总裁,他持有公司股东珠海横琴新区赛普洛投资中心(有限合伙)68.88 万元出资额且直接持有公司股份 1100 股……
值得注意的是,目前 * ST 赛隆第四届董事会共有 9 名董事,此次辞职事件意味着第四届董事会的所有成员均递交了辞职报告。而此次大规模的高层人事变动,根源在于公司控制权的变更。
据悉,7 月 9 日晚间,赛隆药业就曾发布公告称,公司的控股股东已变更为海南雅亿共赢科技合伙企业(有限合伙),并且由于目前海南雅亿无实际控制人,所以公司也随之无实际控制人。在公司控制权发生如此重大变更的背景下,高层管理人员的变动似乎也在情理之中。
从资本市场的反应来看,截至 8 月 11 日收盘,*ST 赛隆股价报 16.18 元 / 股,跌幅达 3.75%,总市值为 28.48 亿元,一定程度上反映了投资者对此次人事变动的担忧。不过,公司也在积极应对这一变化。8 月 10 日,*ST 赛隆召开了第四届董事会第十次会议,审议通过了《关于聘任公司总裁的议案》,同意聘任陈科担任公司总裁,任期自本次董事会会议审议通过之日起至第四届董事会届满之日止。同时,公司还提名了贾晋斌先生、陈科先生、陈顿斐先生、张光扬先生、陈榕辉先生、李童瑶女士为公司第四届董事会非独立董事候选人,新一届独立董事候选人包括王淑芳、张建民、张凯三人。
此次赛隆药业的高层大换血,对于公司未来的发展无疑是一个重大转折点。新的管理层将为公司带来怎样的战略调整和发展思路,公司在业务布局、市场拓展、研发投入等方面是否会有新的举措,这些都充满了不确定性。但可以肯定的是,赛隆药业正站在一个全新的起点上,面临着新的机遇与挑战。
资料显示,赛隆药业是一家集药品研发、生产、销售为一体的企业,目前产品主要集中在神经系统、心脑血管系统和消化系统等领域。近年来,赛隆药业业绩持续承压。数据显示,2020年以来,赛隆药业仅有2023年盈利,其余年份均处于亏损状态。其中2025年第一季度报告显示,赛隆药业实现营业收入同比下降22.2%至5409万元;归母净利润同比下降163.9%,亏损104万元。
业内指出,赛隆药业的情况并非个例。近年来,医药行业高管、核心技术人员的离职现象愈发频繁。而仅在 2025 年 8 月以来,就有不少于 10 家药企发布人事变动公告,涉及副总裁、外部董事等多个关键岗位。
而在 “离职风暴”的背后是多重因素的交织。如医药市场竞争日益激烈,创新药的研发成为药企角逐的关键战场。然而,创新药研发周期长、投入大、风险高,对于管理层的战略眼光、决策能力以及资源整合能力都有着非常高的要求,一些药企管理层在面对激烈竞争和企业发展瓶颈时,感到力不从心,从而选择离开。此外,当下,整个医药行业正处于转型升级的关键时期,从仿制药为主向创新药转型成为大势所趋,药企在转型过程中,需要对组织架构、业务模式、人才结构等进行全面调整。部分高管可能因无法适应企业转型的节奏,或者与新的发展战略理念不合,而主动或被动地离开了公司。
喜欢我们文章的朋友点个“在看”和“赞”吧,不然微信推送规则改变,有可能每天都会错过我们哦~
免责声明
“汇聚南药”公众号所转载文章来源于其他公众号平台,主要目的在于分享行业相关知识,传递当前最新资讯。图片、文章版权均属于原作者所有,如有侵权,请在留言栏及时告知,我们会在24小时内删除相关信息。
信息来源:制药网
往期推荐
本平台不对转载文章的观点负责,文章所包含内容的准确性、可靠性或完整性提供任何明示暗示的保证。
2025-06-18
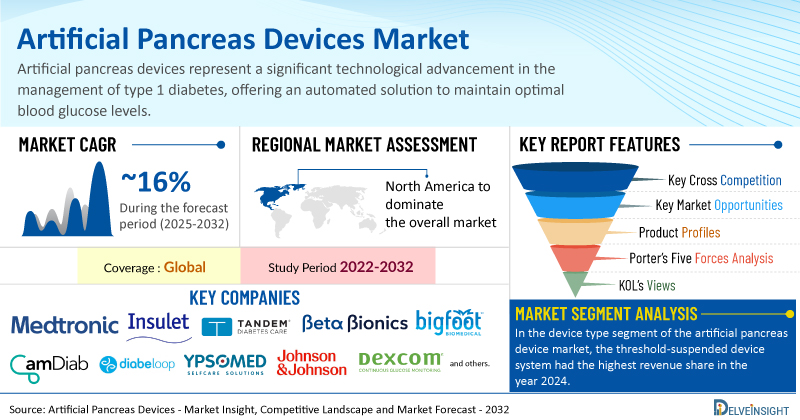
The global surge in diabetes cases is a key factor propelling the artificial pancreas device market, as a larger patient base fuels the need for effective diabetes management tools. Growing awareness and widespread screening initiatives have also contributed to earlier diagnoses and improved disease control, reinforcing the demand for advanced technologies such as artificial pancreas systems. Furthermore, ongoing product innovation and development by leading companies have led to more efficient and accessible devices, boosting market growth.
New York, USA, June 18, 2025 (GLOBE NEWSWIRE) --
Global Artificial Pancreas Devices Market is Gearing Up for Outstanding Expansion at a CAGR of ~16% by 2032 | DelveInsight
The global surge in diabetes cases is a key factor propelling the artificial pancreas device market, as a larger patient base fuels the need for effective diabetes management tools. Growing awareness and widespread screening initiatives have also contributed to earlier diagnoses and improved disease control, reinforcing the demand for advanced technologies such as artificial pancreas systems. Furthermore, ongoing product innovation and development by leading companies have led to more efficient and accessible devices, boosting market growth.
DelveInsight’s
Artificial Pancreas Devices Market Insights
report provides the current and forecast market analysis, individual leading artificial pancreas devices companies’ market shares, challenges, artificial pancreas devices market drivers, barriers, trends, and key market artificial pancreas devices companies in the market.
Key Takeaways from the Artificial Pancreas Devices Market Report
As per DelveInsight estimates, North America is anticipated to dominate the global artificial pancreas devices market during the forecast period.
In the device type segment of the artificial pancreas device market, the threshold-suspended device system had the highest revenue share in the year 2024.
Notable artificial pancreas devices companies such as
Medtronic PLC, Insulet Corporation, Tandem Diabetes Care, Inc., Beta Bionics, Inc., BIGFOOT BIOMEDICAL, INC., CamDiab Ltd., Diabeloop SA, Ypsomed, INREDA® DIABETIC B.V., Nikkiso Co., Ltd., Pancreum, Inc., Johnson & Johnson Services, Inc., Dexcom, Inc., TypeZero Technologies, Animas Corporation,
and several others are currently operating in the artificial pancreas device market.
In
September 2024, Tandem Diabetes Care, Inc.
, announced that its t:slim X2 insulin pump with Control-IQ automated insulin delivery (AID) technology was cleared for use with Eli Lilly and Company’s Lyumjev® (insulin lispro-aabc injection) ultra-rapid acting insulin in the European Union (EU).
In
June 2024, MCRA
, a leading privately held independent medical device, diagnostics, and biologics Clinical Research Organization (CRO) and advisory firm, announced its role in assisting CamDiab's artificial pancreas software, CamAPS FX, in achieving U.S. Food and Drug Administration (FDA) clearance.
To read more about the latest highlights related to the artificial pancreas devices market, get a snapshot of the key highlights entailed in the
Global Artificial Pancreas Devices Market Report
Artificial Pancreas Devices Overview
Artificial pancreas devices represent a significant technological advancement in the management of type 1 diabetes, offering an automated solution to maintain optimal blood glucose levels. These systems typically combine a continuous glucose monitor (CGM), an insulin pump, and a sophisticated control algorithm that calculates and delivers insulin doses in real time. By mimicking some functions of a healthy pancreas, these devices help reduce the burden of frequent glucose monitoring and insulin administration, improving glycemic control and reducing the risk of both hyperglycemia and hypoglycemia.
Recent innovations have led to the development of hybrid closed-loop systems, which require minimal patient intervention and can automatically adjust insulin delivery based on sensor readings. These devices have shown promising results in clinical trials and real-world settings, enhancing quality of life and long-term health outcomes for people with diabetes. Ongoing research is focused on improving algorithm accuracy, integrating dual hormone (insulin and glucagon) systems, and enhancing interoperability with smartphones and wearable technologies to create fully autonomous and user-friendly artificial pancreas solutions.
Artificial Pancreas Devices Market Insights
North America is projected to dominate the artificial pancreas device market in the coming years. This leadership can be attributed to several factors, including the growing prevalence of diabetes, increased government healthcare initiatives, the presence of major industry players engaged in strategic product innovations, and greater public awareness about diabetes management. Notably, rising awareness plays a significant role in driving demand for advanced diabetes care technologies such as artificial pancreas systems. For example, collaborative efforts by the National Institutes of Health (NIH) and the Centers for Disease Control and Prevention (CDC) through programs like the National Diabetes Education Program (NDEP) aim to educate communities about continuous glucose monitoring and automated insulin delivery systems.
Moreover, active product development and regulatory approvals continue to strengthen the market. A key milestone includes the FDA approval in May 2024 of the CamAPS FX artificial pancreas, developed by the University of Cambridge, for use in individuals with type 1 diabetes aged two years and above, including pregnant women.
Collectively, these factors are expected to significantly fuel the growth of the artificial pancreas device market in North America throughout the 2025–2032 forecast period.
To know more about why North America is leading the market growth in the artificial pancreas devices market, get a snapshot of the
Artificial Pancreas Devices Market Outlook
Artificial Pancreas Devices Market Dynamics
The artificial pancreas devices market is witnessing strong growth driven by
increasing global prevalence of type 1 diabetes
and
advancements in diabetes management technologies
. As patient demand for convenient and accurate diabetes management tools rises, healthcare providers and manufacturers are increasingly focusing on
developing integrated, closed-loop systems
that mimic the glucose-regulating function of a healthy pancreas.
Technological innovation is a core driver of the market, with
developments in real-time glucose monitoring, machine learning algorithms, and smartphone integration
enhancing the usability and precision of artificial pancreas systems. Companies are also leveraging cloud connectivity to enable remote patient monitoring and data sharing with healthcare providers. These advancements are improving both patient outcomes and physician decision-making while reducing the burden of disease management. The
adoption of hybrid closed-loop systems
, which allow partial automation, has paved the way for fully automated solutions, many of which are currently in advanced stages of clinical trials or have already gained regulatory approvals in key markets like the U.S. and Europe.
Regulatory support and favorable reimbursement policies
in several countries are also propelling market growth. Additionally, public and private payers are beginning to cover these systems, recognizing their long-term benefits in reducing diabetes-related complications and hospitalizations. However, in developing countries,
market penetration remains limited
due to high device costs and insufficient healthcare infrastructure.
Despite these promising trends, the market faces notable challenges.
High upfront costs of artificial pancreas systems
, coupled with the
need for regular maintenance and consumables
, can deter widespread adoption, especially in low- and middle-income regions. Technical limitations, such as
sensor calibration requirements, occasional device malfunction, and user training complexity
, can also hinder patient acceptance and adherence. Furthermore,
data security and privacy concerns
regarding cloud-connected health devices must be carefully managed to maintain trust among users and healthcare providers.
Looking ahead, the artificial pancreas market is poised for robust growth, supported by continuous innovation, strategic partnerships, and rising consumer awareness. Industry players are investing in R&D to enhance the accuracy, affordability, and user-friendliness of these systems. As the ecosystem evolves, integration with digital health platforms and expansion into emerging markets are expected to open new avenues for growth, ultimately moving closer to the vision of a fully automated, reliable, and accessible solution for diabetes management.
Get a sneak peek at the artificial pancreas devices market dynamics @
Artificial Pancreas Devices Market Trends
Report Metrics
Details
Coverage
Global
Study Period
2022–2032
Artificial Pancreas Devices Market CAGR
~16%
Artificial Pancreas Devices Market Size by 2032
USD 1.1 Billion
Key Artificial Pancreas Devices Companies
Medtronic PLC, Insulet Corporation, Tandem Diabetes Care, Inc., Beta Bionics, Inc., BIGFOOT BIOMEDICAL, INC., CamDiab Ltd., Diabeloop SA, Ypsomed, INREDA® DIABETIC B.V., Nikkiso Co., Ltd., Pancreum, Inc., Johnson & Johnson Services, Inc., Dexcom, Inc., TypeZero Technologies, Animas Corporation, among others
Artificial Pancreas Devices Market Assessment
Artificial Pancreas Devices Market Segmentation
Artificial Pancreas Devices Market Segmentation By Device Type:
Threshold Suspended Device System and Non-Threshold Suspended Device System
Artificial Pancreas Devices Market Segmentation By End User:
Hospitals & Clinics, Homecare Settings, Ambulatory Surgical Centers, and Others
Artificial Pancreas Devices Market Segmentation By Geography
: North America, Europe, Asia-Pacific, and Rest of World
Porter’s Five Forces Analysis, Product Profiles,
Case Studies, KOL’s Views, Analyst’s View
Which MedTech key players in the artificial pancreas devices market are set to emerge as the trendsetter explore @
Artificial Pancreas Devices Companies
Table of Contents
1
Artificial Pancreas Devices Market Report Introduction
2
Artificial Pancreas Devices Market Executive Summary
3
Competitive Landscape
4
Regulatory Analysis
5
Artificial Pancreas Devices Market Key Factors Analysis
6
Artificial Pancreas Devices Market Porter’s Five Forces Analysis
7
Artificial Pancreas Devices Market Layout
8
Artificial Pancreas Devices Market Company and Product Profiles
9
KOL Views
10
Project Approach
11
About DelveInsight
12
Disclaimer & Contact Us
Interested in knowing the artificial pancreas devices market by 2032? Click to get a snapshot of the
Artificial Pancreas Devices Market Analysis
Related Reports
Diabetes Market
Diabetes Market Insights, Epidemiology, and Market Forecast – 2034
report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key diabetes companies, including
TikoMed, Avotres, REMD Biotherapeutics, Novo Nordisk,
among others.
Diabetes Pipeline
Diabetes Pipeline Insight – 2025
report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key diabetes companies, including
Daewoong Pharmaceutical, Janssen Biotech, Zealand Pharma, BioRestorative Therapies, Elevian, Oramed Pharmaceuticals, ImCyse, Novo Nordisk, Enthera, Precigen, Inc., Japan Tobacco, Avotres, AstraZeneca, Landos Biopharma, Vertex Pharmaceuticals, REMD Biotherapeutics, Inc., Eledon Pharmaceuticals, Eli Lilly and Company, Novo Nordisk A/S, Diamyd Medical, NextCell Pharma, ViaCyte, Op-T LLC, Dompé Farmaceutici S.p.A, ILTOO Pharma, Throne Biotechnologies, Oramed Pharmaceuticals, Janssen Research & Development, LLC, Jaguar Gene Therapy, SQZ Biotechnologies, Genprex, Inc., CRISPR Therapeutics, Biora Therapeutics, Genprex, Inc.,
among others.
Type 1 Diabetes Market
Type 1 Diabetes Market Insights, Epidemiology, and Market Forecast – 2034
report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key type 1 diabetes companies, including
Landos Biopharma, Diamyd Medical, Gan&Lee Pharmaceuticals, Zealand Pharma, Kamada, AstraZeneca, Novo Nordisk, Provention Bio Preregistration, Histogen, Vertex Pharmaceuticals, Panbela Therapeutics, Arecor, Bioprojet, Novartis, ImCyse, Adocia, Anelixis Therapeutics, Tolerion, TikoMed, Avotres, REMD Biotherapeutics, Novo Nordisk,
among others.
Type 2 Diabetes Market
Type 2 Diabetes Market Insights, Epidemiology, and Market Forecast – 2034
report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key type 2 diabetes companies, including
Eli Lilly and Company, Regor Pharmaceuticals Inc., AstraZeneca, Eccogene, Pfizer, Sciwind Biosciences USA Co., Ltd., MediciNova, Sparrow Pharmaceuticals, HighTide Biopharma Pty Ltd, Novo Nordisk A/S, Biomea Fusion Inc.,
among others.
Type 2 Diabetes Pipeline
Type 2 Diabetes Pipeline Insight – 2024
report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key type 2 diabetes companies, including
Tonghua Dongbao Pharmaceutical, Eli Lilly and Company, Rivus Pharmaceuticals, Celon Pharma, Sciwind Biosciences, AstraZeneca, Suzhou Alphamab Co., Ltd., Neurodon, Abarceo Pharma, Chong Kun Dang Pharmaceutical,
among others.
DelveInsight’s
Pharma Competitive Intelligence Service
:
Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Pipeline Assessment
Healthcare Licensing Services
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our
Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant, and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance.
Contact Us
Shruti Thakur
info@delveinsight.com
+14699457679
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability
for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this
article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
100 项与 Celon Pharma SA 相关的药物交易
登录后查看更多信息
100 项与 Celon Pharma SA 相关的转化医学
登录后查看更多信息
组织架构
使用我们的机构树数据加速您的研究。
登录
或

管线布局
2026年02月28日管线快照
管线布局中药物为当前组织机构及其子机构作为药物机构进行统计,早期临床1期并入临床1期,临床1/2期并入临床2期,临床2/3期并入临床3期
药物发现
2
12
临床前
临床1期
2
3
临床2期
其他
15
登录后查看更多信息
当前项目
登录后查看更多信息
药物交易
使用我们的药物交易数据加速您的研究。
登录
或

转化医学
使用我们的转化医学数据加速您的研究。
登录
或

营收
使用 Synapse 探索超过 36 万个组织的财务状况。
登录
或

科研基金(NIH)
访问超过 200 万项资助和基金信息,以提升您的研究之旅。
登录
或

投资
深入了解从初创企业到成熟企业的最新公司投资动态。
登录
或

融资
发掘融资趋势以验证和推进您的投资机会。
登录
或

生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用

