预约演示
更新于:2025-05-07
CD40L x TLR4 x CD70
更新于:2025-05-07
关联
3
项与 CD40L x TLR4 x CD70 相关的药物作用机制 CD40L刺激剂 [+2] |
在研机构 |
在研适应症 |
非在研适应症- |
最高研发阶段临床前 |
首次获批国家/地区- |
首次获批日期1800-01-20 |
作用机制 CD40L刺激剂 [+2] |
在研机构- |
在研适应症- |
非在研适应症 |
最高研发阶段终止 |
首次获批国家/地区- |
首次获批日期1800-01-20 |
作用机制 CD40L抑制剂 [+2] |
在研机构- |
在研适应症- |
最高研发阶段无进展 |
首次获批国家/地区- |
首次获批日期1800-01-20 |
9
项与 CD40L x TLR4 x CD70 相关的临床试验JPRN-jRCT2073210013
Autologous Dendritic Cell Vaccine Therapy Targeting HTLV-1 Specific Antigen for Adult T-Cell Leukemia/Lymphoma A Phase II multicenter randomized controlled Study - Autologous DC Vaccine Therapy Targeting HTLV-1 Specific Antigen for ATLL A Phase II Study
开始日期2021-04-20 |
申办/合作机构- |
NCT02888756
A Phase IIa Randomized, Placebo Controlled, Double Blinded Study to Evaluate the Safety and Immunogenicity of iHIVARNA-01 in Chronically HIV-infected Patients Under Stable Combined Antiretroviral Therapy
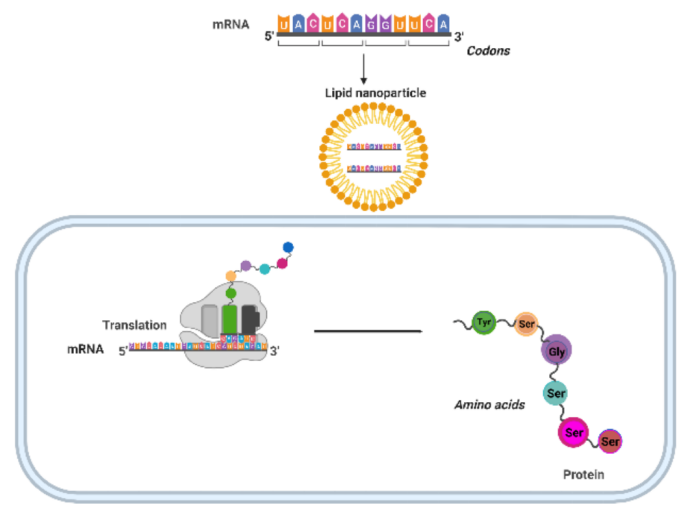
iHIVARNA-01 is a novel therapeutic vaccine for the treatment of HIV-1-infected patients based on in vivo modification of DCs. It consists of HIVACAT-TriMix: mRNA encoding a mixture of APC activation molecules (CD40L, a constitutively active variant of TLR4 and CD70) and the HIV target antigens contained in HIVACAT to be administered through the intranodal route. iHIVARNA-01 aims to achieve the 'functional cure' of HIV infection, i.e. controlling viral replication in the absence of anti-retroviral therapy.
开始日期2017-04-04 |
申办/合作机构 |
100 项与 CD40L x TLR4 x CD70 相关的临床结果
登录后查看更多信息
100 项与 CD40L x TLR4 x CD70 相关的转化医学
登录后查看更多信息
0 项与 CD40L x TLR4 x CD70 相关的专利(医药)
登录后查看更多信息
8
项与 CD40L x TLR4 x CD70 相关的文献(医药)2016-02-01·Cancer Immunology Research1区 · 医学
Intratumoral Delivery of TriMix mRNA Results in T-cell Activation by Cross-Presenting Dendritic Cells
1区 · 医学
Article
作者: Breckpot, Karine ; Goethals, Lode ; Bialkowski, Lukasz ; Renmans, Dries ; Goyvaerts, Cleo ; Broos, Katrijn ; Du Four, Stephanie ; Benteyn, Daphné ; Maenhout, Sarah ; Van der Jeught, Kevin ; Thielemans, Kris ; Flamand, Véronique ; Heirman, Carlo ; Van Lint, Sandra
2013-10-01·Annals of Oncology1区 · 医学
A phase IB study on intravenous synthetic mRNA electroporated dendritic cell immunotherapy in pretreated advanced melanoma patients
1区 · 医学
Article
作者: Aerts, C ; Thielemans, K ; Wilgenhof, S ; Van Nuffel, A M T ; Van Riet, I ; Neyns, B ; Benteyn, D ; Heirman, C ; Corthals, J ; Bonehill, A
2013-08-15·The Journal of Immunology2区 · 医学
Modulation of Regulatory T Cell Function by Monocyte-Derived Dendritic Cells Matured through Electroporation with mRNA Encoding CD40 Ligand, Constitutively Active TLR4, and CD70
2区 · 医学
Article
作者: Pen, Joeri J ; De Keersmaecker, Brenda ; Heirman, Carlo ; Thielemans, Kris ; Van Nuffel, An M T ; Corthals, Jurgen ; Aerts, Joeri L ; Bonehill, Aude ; Escors, David ; Maenhout, Sarah K ; Breckpot, Karine
6
项与 CD40L x TLR4 x CD70 相关的新闻(医药)2024-09-19
摘要:mRNA疫苗作为有前景的癌症疗法已经发展起来。这些疫苗可以编码与肿瘤相关的抗原,从而实现个性化的治疗方法。它们还可以针对癌症特有的突变,并克服免疫逃逸机制。它们通过操纵身体的细胞功能来产生抗原,引发免疫反应,并通过克服与特定组织相容性白细胞抗原分子相关的限制来抑制肿瘤。然而,成功地将mRNA传递到目标细胞是一个关键挑战。病毒和非病毒载体(脂质纳米颗粒和阳离子脂质体)在保护mRNA免受破坏和协助细胞摄取方面显示出了巨大的能力。细胞穿透肽、水凝胶、基于聚合物的纳米颗粒和树状分子已被研究以增加mRNA的传递效率和免疫原性。这篇全面的综述探讨了mRNA疫苗及其用于癌症的传递平台,涉及设计考虑、多种传递策略和最新进展。总体而言,这篇综述为mRNA疫苗作为有效癌症治疗的创新策略的进展做出了贡献。
1.引言
mRNA疫苗领域在mRNA疫苗成功对抗传染病之后经历了突破性的进步。mRNA疫苗在提供针对病毒病原体的特异性和引人注目的免疫反应方面的空前成功,激发了对mRNA治疗癌症疗法的强烈研究努力。癌症疫苗历来包括与癌症相关的抗原(CAAs)、免疫刺激剂和癌症特异性抗原(Ags)。与传统疫苗相比,它们提供了多种优势,如编码多种蛋白质、引发强大的免疫反应以及促进快速开发和生产。mRNA疫苗可以表达包含众多表位的完整Ags,同时绕过主要组织相容性复合体(MHC)的限制。然而,将mRNA疫苗传递到目标细胞面临着重大挑战。
mRNA疫苗的成功在很大程度上取决于推进有效的传递平台,这些平台确保mRNA的稳定性、保护和有效的细胞内传递。各种传递平台被开发出来,以提高mRNA疫苗的效力,同时最小化潜在的不良反应。基于脂质的纳米颗粒(LNPs)在mRNA疫苗开发中至关重要,它们可以成功地封装和保护mRNA分子,促进细胞摄取,实现内质网逃逸,并帮助有效地转化为Ags。脂质化学和配方技术的最新进展显著提高了LNPs的效力,导致在临床前和临床环境中成功传递mRNA。
病毒载体长期以来被用于基因传递,并作为mRNA疫苗载体获得了相当大的关注。这些载体可以有效地转导目标细胞,实现mRNA的细胞内传递以进行后续翻译。病毒载体提供高转导效率,并可以触发强大的免疫反应。然而,安全问题、预先存在的免疫力和有限的货物容量提出了挑战,这些挑战必须得到解决,以便它们在mRNA疫苗传递中的成功应用。
基于聚合物的载体作为mRNA传递平台被探索,并由于其多功能性、生物相容性和可调节属性提供了多种优势。它们与细胞膜的有害电荷组分进行静电连接,帮助细胞摄入并促进从内质网转移。此外,将靶向配体整合到聚合物载体上允许特定部位的传递和提高效力。基于肽的传递平台作为mRNA传递平台得到了发展,它们利用其低毒性、合成简便和潜在的靶向传递能力。肽可以被设计成具有固有的细胞穿透属性或针对特定细胞表面受体,促进有效的mRNA摄取和细胞内传递。细胞穿透的、pH响应的和自组装肽在增强mRNA疫苗传递和随后Ags表达方面显示出了有希望的结果。这篇综述讨论了不同mRNA疫苗在临床前和临床开发中的现状。它通过关注关键考虑因素,如mRNA修饰、Ags选择和佐剂整合,来优化它们的设计。此外,它探讨了用于将mRNA传递到目标细胞的多种策略,包括病毒载体和基于脂质的纳米结构、基于聚合物的载体、基于肽的支架系统和纳米材料。此外,这篇综述强调了最近的进展和创新方法,如树状分子和离子电渗技术,旨在克服与mRNA疫苗传递相关的挑战。这些进步旨在提高mRNA疫苗传递平台的安全性、效力和转化潜力。我们的综述最后通过追踪mRNA疫苗及其传递策略的演变,从它们在传染病和癌症治疗中的成功应用开始。这篇综述为mRNA疫苗和先进的传递平台提供了深入的分析,为这一有前景的领域的当前状态和前景提供了宝贵的见解。
2.mRNA疫苗
RNA分子作为一类治疗药物获得了显著的认可,尤其是在成功开发2019冠状病毒病(COVID-19)疫苗之后。这一突破为针对传统上具有挑战性和“不可成药”的目标以及在基因层面治疗疾病开辟了新的可能性。mRNA疫苗代表了一种有前景的癌症治疗方法,提供了特定且耐受性良好的治疗选择,已在临床前研究中显示出对实体肿瘤的有效性。例如,采用生物信息学驱动的策略开发了一种专门针对卵巢癌和乳腺癌中的癌症抗原125(CA-125)的mRNA疫苗。疫苗设计包括自佐剂特性和计划增加树突状细胞(DCs)抗原呈递能力的元素。检测到干扰素-γ(IFN-g)和分化簇8阳性(CD8+)T细胞激活,验证了疫苗的潜力。为了获得强大的抗肿瘤效果,必须有效地传递和翻译编码肿瘤抗原的mRNA,并同时触发先天免疫反应以促进抗原呈递。因此,针对DCs是提高疫苗效率的有利方法。目前,已经探索了多种策略来特别针对DCs。一种方法涉及使用抗原传递载体,包括纳米颗粒或脂质体,这些载体装饰有与DC受体、DC-SIGN或CD40特异性相互作用的配体。这些配体促进DCs选择性地摄取抗原,促进抗原呈递给T细胞。此外,在疫苗配方中加入佐剂可以激活DCs,进一步增强其刺激免疫反应的能力。这种针对性疫苗在提高包括癌症和传染病病原体在内的各种疾病的疫苗精确度和效力方面具有巨大潜力。这一进展是治疗性癌症疫苗研究的一个重要里程碑。表1总结了不同的mRNA疫苗、目标抗原和临床前及临床试验的关键发现(表1)。
2.1.mRNA疫苗在临床前试验中
众多的临床前研究已经调查了针对多种类型癌症的各种mRNA疫苗。最近的研究调查了肿瘤衍生RNA作为设计基于树突细胞(DC)疫苗的抗原来源及其在肿瘤疫苗策略中的有效性。Kyte等人进行了自体肿瘤mRNA树突细胞(tDCs)作为针对黑色素瘤的疫苗的临床前评估。tDCs诱导了对肿瘤特异性抗原的T细胞反应。该研究表明tDC特异性反应,包括所有六名患者中的T细胞增殖,在两名患者中分泌干扰素-γ(IFN-g),以及一名患者中HLA的阻断。此外,Kyte等人证明tDC疫苗无毒性,并成功在黑色素瘤患者中诱发T细胞反应。Key等人的另一项研究旨在评估有趣的T细胞反应并调查它们作为加强剂量的持续长期效力。他们展示了针对抗原的显著细胞介导的免疫反应。此外,研究确认即使在来自单一细胞的T细胞克隆中,辅助性T细胞1/2(Th1/Th2)细胞因子谱也是混合的。这表明疫苗本身可能不足以作为单一疗法,并为其与免疫检查点抑制剂(ICIs)的结合提供了强有力的理由。这项评估突出了这种组合作为恶性黑色素瘤疫苗策略的潜在用途。
佐剂用于选择性激活不同的免疫组分,从而增强疫苗的免疫刺激性活性。mRNA疫苗中使用的佐剂可分为三组:(1)具有自我佐剂特性的RNA,(2)传递系统的组成部分,以及(3)外部免疫刺激剂。临床前调查提供了证据,证实了将ICIs和癌症疫苗结合用于非免疫原性肿瘤治疗的使用。这种方法在免疫治疗领域呈现了一个有希望的途径。Liu等人合成了带有血凝素(HA)标签的黏蛋白1(MUC1)mRNA,以制定mRNA疫苗。使用脂质/钙/磷酸盐(LCP)系统作为佐剂开发mRNA疫苗。在这项研究中,评估了编码MUC1新抗原的mRNA疫苗的有效性,旨在激活位于淋巴结内的DCs。研究展示了成功的T细胞刺激和扩增,特别是针对三阴性乳腺癌(TNBC)肿瘤。为了进一步提高mRNA疫苗的抗肿瘤效力,它与明确针对细胞毒性T淋巴细胞蛋白4(CTLA-4)的抗体结合使用。疫苗诱发了强大且针对性的CTL反应,疫苗和特定CTLA-4抗体的同时给药显著增强了对肿瘤的免疫反应。Gu等人调查了通过将Toll样受体(TLR)2/6 mRNA,特别是Pam2Cys(一种佐剂),纳入脂质纳米颗粒(LNPs)来增强mRNA疫苗效力。用这种疫苗进行免疫调节了引流淋巴结(dLNs)中的免疫微环境,导致包括白介素-12(IL-12)和IL-17在内的关键细胞因子的释放。抗原呈递主要由迁移性和dLN驻留的传统2型DCs促进,导致CD4+和CD8+ T细胞介导的强大抗肿瘤反应。
在另一项研究中,Tse等人证明编码构成型激活的干扰素基因刺激物V155M(STINGV155M)蛋白的mRNA作为增强CD8+ T细胞反应的有效遗传佐剂。疫苗诱导的干扰素刺激反应元件和核因子kB途径激活了I型干扰素反应,并增强了抗原特异性T细胞。用编码E6和E7肿瘤蛋白和STINGV155M的mRNA进行疫苗接种,导致人乳头瘤病毒阳性(HPV+)TC-1肿瘤小鼠模型中肿瘤生长减少。这项研究为mRNA编码佐剂在增强疫苗效力方面的有效性提供了令人信服的证据。
另一种方法涉及将mRNA疫苗NP与佐剂C16-R848结合使用,这增强了合成mRNA疫苗在癌症治疗中的效力。这种由编码卵清蛋白(OVA)的mRNA和带有C16-R848涂层的脂质-聚乙二醇(PEG)壳组成的配方,保留了佐剂的活性。它显著提高了mRNA转染效率,并通过MHC类I促进了抗原的呈递。这种方法通过增强OVA特异性CD8+ T细胞的增殖及其在肿瘤床中的积累,刺激了有效的T细胞介导的免疫反应。张和夏开发了一种自佐剂mRNA纳米疫苗用于癌症治疗。这种纳米疫苗由编码肿瘤抗原的mRNA和类似脂质的材料(C1)组成。它有效地将mRNA传递给DCs,并同时刺激TLR4,从而激活T细胞。重要的是,这种纳米疫苗在几个小鼠模型中显示出显著的抗肿瘤效果。
TriMix由CD40L和CD70 mRNA以及TLR4组成,在晚期黑色素瘤中展示了佐剂活性。TriMix和TriMixDC-MEL的配方,由自体单核细胞衍生的mRNA共电穿孔DCs组成,在晚期黑色素瘤中展现了安全性和抗癌效果。Van Lint等人调查了CAA mRNA(酪氨酸酶相关蛋白-2 [Trp2]、Wilms肿瘤基因1 [WT1]或酪氨酸酶)与佐剂TriMix的淋巴结内递送。这种方法诱导了DCs的成熟,促进了LNs内CAA特异性T细胞的激活。研究表明选择性地摄取mRNA疫苗,导致CD11c+细胞在LNs中编码Trp2、WT1或酪氨酸酶。TriMix mRNA的存在创造了一个吸引和刺激CD4+和CD8+ T细胞的环境,特别是针对抗原。此外,这种疫苗在诱导CTLs和在小鼠模型中产生治疗反应方面显示出与mRNA电穿孔DCs相当的效力。这项研究强调了淋巴结内递送CAA mRNA,结合免疫调节mRNA作为一种有希望的疫苗策略的潜力。
Ramos da Silva等人进行了一项研究,涉及将自扩增mRNA以及未修饰和核苷修饰的非复制型mRNA封装进脂质纳米颗粒(LNPs)中,以开发疫苗。这些疫苗编码一种嵌合蛋白,包括HPV-16 E7肿瘤蛋白和1型单纯疱疹病毒糖蛋白D(gDE7)。该研究发现,即使是单次低剂量免疫接种这些gDE7 mRNA疫苗中的任何一种,都能激活特异性E7 CD8+ T细胞。此外,这些疫苗诱导的记忆T细胞反应能够预防肿瘤复发,并在不同生长阶段有效消除皮下肿瘤。此外,gDE7 mRNA-LNP疫苗在单剂量疫苗后,对同种异体小鼠肿瘤模型显示出显著的肿瘤保护效力。比较分析强调了这些mRNA-LNP疫苗比gDE7 DNA和gDE7重组蛋白疫苗的优越性。这项研究强调了基于mRNA的疫苗在癌症治疗免疫疗法中的潜力。
2.2.处于临床试验阶段的mRNA疫苗
最近的研究评估了使用mRNA疫苗针对癌症的可行性。在临床治疗的子宫癌患者中,给予含有WT1 mRNA的自体DC基础疫苗。HLA-A2阳性的患者观察到积极的肿瘤学和免疫学反应,而HLA-A2阴性的患者没有表现出这种反应。另一项临床试验涉及使用来自自体癌症干细胞的mRNA的单核细胞衍生的自体DC基础疫苗。接种疫苗的患者显示出强大的免疫反应和延长的无进展生存期。用剂量强度的替莫唑胺和编码pp65溶酶体相关膜糖蛋白的DC基础mRNA疫苗治疗胶质瘤患者,显示出延长的无进展生存期和增加的pp65细胞反应。Cafri等人开发了mRNA-4650,它编码自体癌症抗原。该疫苗包含来自患者肿瘤的肿瘤蛋白53(TP53)、KRAS或磷脂酰肌醇3激酶、催化亚单位α(PIK3CA)突变,以及多达15个预测的I类HLA新抗原。mRNA-4650在I/II临床阶段是安全的,并触发了针对预期新表位的突变特异性T细胞反应。由携带mRNA编码的肿瘤抗原癌症睾丸(CT)7、黑色素瘤抗原基因(MAGE)-A3和WT1的朗格汉斯型DC(LCs)组成的疫苗,通过抗原特异性CD4和CD8 T细胞诱导了干扰素-γ(IFN-γ)、白介素-2(IL-2)和肿瘤坏死因子(TNF)-α的释放,以及接种组内的克隆扩增。在临床研究中,基于DC的mRNA疫苗在多种癌症类型中显示出有希望的免疫反应和改善的生存期。
BNT162b2是一种核苷修饰的RNA和含有LNP的疫苗。Shroff等人比较了接受细胞毒性治疗的癌症患者和对照组对BNT162b2 mRNA疫苗的反应。首剂后,67%的癌症患者产生了中和抗体,第二剂后增加了3倍。尽管癌症患者显示出针对Spike蛋白的抗体和T细胞,但他们的反应比对照组弱。在癌症患者中识别出特定于Spike受体结合域的记忆B细胞亚群,可能预示着对额外剂量的更好反应。一项针对20名癌症患者的I期研究发现,给予第三剂疫苗使中和抗体增加了3倍,伴随轻微的不良反应,但没有T细胞改善。Harrington等人评估了16名慢性髓性白血病(CML)患者接受BNT162b2疫苗单剂量后的免疫效应,这些患者之前接受了酪氨酸激酶抑制剂治疗。在接种疫苗后21天内,87.5%的CML患者表现出抗Spike免疫球蛋白G(IgG),产生了中和抗体。在可评估的患者中,93.3%检测到T细胞反应,80%表现出多功能性反应,包括CD4+和CD8+ T细胞。慢性淋巴细胞性白血病/小淋巴细胞性淋巴瘤(CLL/SLL)患者在2剂BNT162b2 mRNA疫苗接种后没有显示出体液反应。然而,近四分之一的人对第三剂疫苗有积极反应。在接受积极治疗的个体和在接种前1年内接受抗CD20治疗的个体中,抗体反应率较低。Thakkar等人发现,第三剂BNT162b2疫苗在最初血清阴性的癌症患者中诱导了57%的血清转换。这种反应保持强劲,抗体水平、T细胞活性和中和能力持续6个月。在接受第三剂疫苗后没有产生足够反应的严重免疫受损的血液恶性肿瘤患者中,临床试验中的第四剂疫苗在67%的患者中增强了免疫力。然而,BNT162b2在不同队列中的免疫原性表现出差异。在骨髓增生性恶性肿瘤患者中反应强劲,达到88%,但多发性骨髓瘤患者表现出明显较低的反应,尤其是那些接受基于抗CD38的治疗的患者。总之,基于LNP的BNT162b2疫苗对接受积极化疗的癌症患者似乎是安全和有益的。
将编码抗原的mRNA和佐剂纳入阴离子脂质体配方可以增强mRNA疫苗的有效性。Tockary等人将RNA工程化,将佐剂与编码抗原的mRNA链结合起来。最终,双链RNA(dsRNA)-连接的mRNA被装载进脂质体配方中,有效地诱导了小鼠和人类DCs,导致大量促炎细胞因子的分泌。OVA mRNA配方已在临床上评估,并在小鼠淋巴瘤(E.G7-OVA)模型中取得了显著的治疗效力。精蛋白是一种小的阳离子肽,作为药物递送候选物质显示出前景。在自然界中,它通过静电键与DNA形成复合物,在精子发生过程中保护DNA的完整性。科学家采用了这种自然方法来开发药物递送平台,使用精蛋白作为RNA载体。在一项显著的应用中,为转移性黑色素瘤开发了一种受精蛋白保护的mRNA疫苗。这种疫苗编码多种肿瘤抗原,导致在I/II期疫苗接种试验中疫苗诱导的T细胞增加和免疫抑制细胞减少。RNActive由CureVac开发,使用编码OVA的修饰mRNA与精蛋白结合,赋予自我佐剂活性,触发针对癌症抗原的免疫反应。这一过程涉及TLR4的激活。RNActive疫苗可以引发强大的免疫反应,包括体液和细胞成分。它们还可以激活包括Th1和Th2细胞在内的耐药细胞亚群,这些细胞在协调免疫反应中至关重要。此外,这些疫苗可以触发短期效应反应和长期记忆反应。基于RNActive技术的mRNA疫苗CV9202显示出固有的自我佐剂特性。它可以针对非小细胞肺癌中常见的六种抗原,并刺激针对广泛CAAs的免疫反应。
CV9103和CV9104是基于RNActive技术开发的抗癌疫苗,专门设计用于针对被诊断为前列腺癌(PC)的个体的靶向治疗。在CV9103在I/II期试验成功后,目前正在对特定的去势抵抗和高风险PC患者进行CV9104作为术前新辅助治疗的临床评估。RNActive基础疫苗CV9201旨在治疗非小细胞肺癌(NSCLC)。它携带表达在NSCLC中的五种抗原的遗传信息。在一项I/IIa期剂量递增研究中,该疫苗显示出有希望的反应,在治疗后检测到免疫反应,支持进一步的临床研究。
最近,IIb期KEYNOTE-942/mRNA-4157-P201试验的积极结果代表了黑色素瘤治疗的重要进展。这项研究调查了Moderna的mRNA-4157个性化癌症疫苗,该疫苗包括一个合成的mRNA,编码34个新抗原,根据患者肿瘤的独特突变特征定制。它与Keytruda(pembrolizumab)联合作为辅助治疗,在完全切除后的III/IV期黑色素瘤患者中使用。
mRNA-4157/V940是一种开创性的基于mRNA的个性化癌症疫苗,专门针对每位患者量身定制,根据他们肿瘤的突变特征编码34个新抗原。当给药时,这种合成mRNA触发适应性免疫反应,为针对患者肿瘤突变的高度靶向的抗肿瘤反应做好准备。
将mRNA-4157/V940与Keytruda(一种增强免疫系统检测和对抗肿瘤细胞能力的免疫疗法)结合使用,预计将通过促进T细胞介导的肿瘤细胞破坏提供额外的好处。试验的突出成就是与单独使用Keytruda相比,复发或死亡的显著减少(44%)。这标志着首次证明由于程序性细胞死亡蛋白1(PD-1)阻断,高风险黑色素瘤患者的无复发生存期得到改善,并表明个性化新抗原方法在革新癌症免疫疗法中的潜力。mRNA-4157/V940的不良事件与早期I期试验一致,Keytruda的安全性概况保持一致。总之,这些开创性的结果突出了像mRNA-4157/V940这样的个性化mRNA癌症疫苗与Keytruda等免疫疗法结合使用的前景,以推进黑色素瘤治疗。它们强调了定制化新抗原方法在癌症免疫疗法中的重要性,为改善患者结果和癌症斗争中的生存带来了希望。
3.mRNA疫苗传递平台
正在评估几种mRNA疫苗技术以引发靶向免疫反应。最近的文献综述探讨了将mRNA治疗剂纳入创新纳米级配方以增强癌症免疫疗法的潜力。此外,一项全面的综述检查了各种专注于提高基于mRNA的癌症疫苗效力的策略。这些综述强调了使用mRNA技术进行癌症治疗和免疫疗法的持续研究和兴趣。
基于现有科学数据,mRNA疫苗技术大致可分为两组:裸mRNA传递方法,包括电穿孔和离子电渗(IP)技术;以及靶向传递系统,包括基于脂质的传递系统,如脂质纳米颗粒(LNPs)和脂质体;聚合物纳米系统,如胶束、树状分子和聚合物水凝胶;基于肽的传递系统;基于碳纳米材料的传递系统;以及病毒载体介导的传递系统。选择这些标准是为了确保对mRNA疫苗传递格局进行全面准确的概述,从而在这个关键领域做出有力的结论和明智的决策。
3.1.裸mRNA传递方法
直接使用mRNA在DCs中产生T细胞反应(抗原特异性)并创造能够消除癌细胞的防御性抗体。通过使用电穿孔将mRNA传递给患者的DCs,这些疫苗技术有望增强免疫系统识别和靶向肿瘤细胞的能力。在结直肠癌的背景下,一项研究对疫苗接种方案进行了比较。具体来说,这项研究评估了两种不同方法的有效性:一种是使用与HLA-A2结合的源自癌胚抗原(CEA)的肽段脉冲的DCs,另一种是使用CEA mRNA电穿孔的DCs。分析表明,使用CEA肽段脉冲DCs有效地刺激了针对结直肠癌的特异性免疫反应。使用针对晚期黑色素瘤患者的单核细胞衍生DCs的mRNA疫苗,显示出显著的CD8+ T细胞反应。同样,将编码WT1抗原的自体成熟mRNA电穿孔DCs皮内注射给急性髓系白血病(AML)患者,也显示出积极的结果。含有自体DCs电穿孔自体癌症mRNA的黑色素瘤疫苗诱导了针对抗原的强烈免疫反应,表明其在体内引发免疫反应的潜力。从人类CD34+前体获得的LCs并用电穿孔WT1 mRNA,显示出持续呈现抗原肽段并诱导强烈的自体CTLs。这种方法消除了对外部细胞因子的需求,展示了mRNA电穿孔LCs作为癌症免疫疗法疫苗的潜力。然而,mRNA基治疗的更广泛应用面临着与它们对核酸酶的敏感性、进入抗原呈递细胞的细胞内传递以及免疫共刺激相关的挑战。克服这些挑战需要确保在传递过程中的稳定性和保护的靶向传递系统。为了推进这个领域,许多研究集中在开发针对癌症治疗背景下mRNA疫苗的靶向传递策略。
最近的一项综述提供了对合成RNA修饰的尖端方法的见解。这些创新方法主要关注核苷酸的改变,包括在50 UTR和30 UTR中纳入假尿苷以及RNA甲基化。这些修饰已被证明显著增强了基于RNA的疫苗的稳定性和效力。通过提高合成RNA的耐用性和性能,这些方法在推进疫苗开发和分子治疗领域具有巨大的潜力。这个研究方向强调了核苷酸工程在优化基于RNA的干预措施结果中的作用。
将IP技术与经皮传递系统相结合,成功地给药了最少的编码gp10025-33肽(KVPRNQDWL)的mRNA疫苗。IP刺激的mRNA疫苗传递引发了免疫反应并激活了皮肤驻留的免疫细胞。这种组合导致了免疫系统的强烈刺激,显著抑制了植入黑色素瘤细胞的小鼠中的肿瘤细胞。它还上调了IFN-γ并增加了肿瘤内CD8+ T细胞的浸润,从而有助于清除肿瘤。研究证明了IP用于传递mRNA疫苗的成功,对黑色素瘤治疗具有潜在的影响。
3.2.基于脂质的纳米系统用于mRNA疫苗传递
LNPs已被记录为mRNA疫苗的多功能传递平台。这些LNPs具有多个优点,包括易于组合和成分增强传递效率及最小化毒性。此外,通过包含佐剂或免疫细胞靶向配体,实现了对免疫反应的定制。LNPs目前在临床应用中最为先进。然而,这些系统涉及多个组分,并引起必须最小化以确保安全的炎症效应。近年来,有几项研究解决了提高LNP安全性的问题。这些努力包括开发可生物降解的LNPs,工程化具有不同脂质样分子的阳离子LNPs,设计增强内质网逃逸的LNPs,以及配制聚乙二醇化LNPs以减少不良免疫反应。此外,具有与环胺辅助的头基的环保阳离子脂质,已被证明可以提高LNPs的有效性和保护力。此外,基于交替共聚物的低炎症mRNA癌症疫苗载体PHTA展示了强大的抗肿瘤免疫反应及最小的炎症副作用,使其成为开发mRNA癌症疫苗的可能策略。
LNP颗粒的大小显著影响其免疫原性。包括稳定性和对目标蛋白的亲和力在内的多种因素,可以影响依赖LNP的RNA传递。例如,带有阳离子基团的脂质促进与内质网膜的静电相互作用,并使疫苗释放到细胞质中。此外,磷脂和聚乙二醇脂质共同促进了LNP介导的RNA传递的有效性。它们影响LNP在体内的清除和分布,进而影响基于LNP的RNA传递系统的性能。此外,包括像C12-200这样的立体纯衍生物在内的阳离子脂质可以影响LNPs的mRNA传递效率,这是由于它们的立体化学。此外,胆固醇和辅助脂质的结合显著促进了使用LNPs进行mRNA转染的有效性。因此,这些不同的脂质组分对于定制LNPs以有效传递RNA至关重要。
在四个LNP组分中,阳离子脂质起着基本作用。这些脂质在其常规pKa下带有正电荷,这使得在自组装过程中加载负电荷RNA到LNPs中成为可能。重要的是,它们在中性pH下保持不变,这有助于最小化毒性并保持LNPs在储存和传递过程中的稳定性。细胞摄取后,随着内质网pH的降低,这些LNPs重新获得正电荷,这有助于它们从内质网逃逸。磷脂中的乙醇胺基团由于其融合特性,可以增强内质网逃逸。此外,LNPs中磷脂的选择可以影响其传递特性。观察到中性磷脂主要促进肝脏传递,而带有负电荷的磷脂改变了LNPs从肝脏到脾脏的靶向。这种器官靶向的选择性可能是设计治疗应用RNA传递系统的有价值特性。
胆固醇通常被纳入作为调节LNP物理性质和纳米结构的调节剂,增强细胞内传递和体内LNP稳定性。结合聚乙二醇化脂质可能增强LNPs的胶体稳定性,延长它们的循环时间。同时,这可以减少它们被细胞摄取并阻碍它们从内质网释放。
通过使用微流体系统,可以制造精确大小的NPs,实现高效RNA封装,以高效率传递。在一项研究中,LNPs在保持一致的脂质组成的情况下系统地修改了大小。有趣的是,小鼠中较小的LNPs显示出显著较低的免疫原性,包括减少的免疫反应,包括抗体产生、免疫细胞激活和记忆细胞的产生。这些发现强调了优化LNP大小以最大化mRNA疫苗治疗效果的重要性。
LNP配方作为mRNA疫苗传递载体显示出显著潜力,证明了其引发强大CTL反应的能力。LNP介导的传递方法涉及分离患者的自体DCs及其随后与编码CAAs的mRNA转染。许多基于脂质的NPs正在作为mRNA疫苗载体进行调查,从而突出了一个积极的研究和开发领域。范等人调查了阳离子脂质辅助NPs(CLAN)作为mRNA疫苗载体。他们的发现揭示了CLAN有效地触发了DC成熟和抗原特异性T细胞增殖。此外,使用OVA mRNA封装的CLAN对携带淋巴瘤的小鼠进行静脉免疫接种,诱导了强大的OVA特异性T细胞反应,减少了肿瘤生长。这些发现突出了CLAN作为mRNA疫苗传递平台的有效性。
在一项相关研究中,一种名为C1脂质(C-12)的阳离子脂质类似化合物有效地将mRNA传递给抗原呈递细胞(APCs),同时激活TLR4并促进强大的T细胞激活。C1脂质可以通过吞噬作用促进mRNA疫苗摄取。此外,C1 LNP可以通过激活DC中的TLR4信号通路,刺激IL-12(一种炎症细胞因子)的表达。值得注意的是,依赖C1脂质的mRNA疫苗在癌症预防和治疗疫苗背景下展示了强大的抗肿瘤反应。此外,这些纳米复合物通过激活细胞质和内质网中的模式识别受体,诱导了先天DC免疫反应。它们还诱导了具有显著Th1特征的DC介导的适应性免疫。这项研究展示了阳离子脂质作为mRNA疫苗传递系统平台的潜力。
此外,一种针对淋巴结(LN)的LNP,称为113-O12B,增强了mRNA癌症疫苗的传递。它还增加了LN中的mRNA表达,并显著改善了对mRNA编码抗原的细胞介导的免疫反应。与PD-1抗体结合使用的LN靶向LNP系统在肿瘤模型中展示了改进的保护和治疗效果,展现了其作为mRNA疫苗多功能平台的潜力。
另外,一种包含棕榈酸-R848(TLR7/8的激动剂)的佐剂脉冲mRNA疫苗NPs的配方,展示了改善的mRNA转染效率和强大的获得性免疫反应。这种配方促进了特定抗原T细胞向肿瘤部位的渗透。它对淋巴瘤和前列腺癌模型有效,预防肿瘤生长和抑制已建立的肿瘤。因此,这种方法为开发有效的癌症免疫疗法提供了希望。
装载编码癌症相关抗原(CAAs)的mRNA的LNP显著减少了肿瘤,并在黑色素瘤模型中延长了总生存期。进一步包含脂多糖(LPS)增强了LNPs的免疫反应。包含修饰的N1-甲基假尿苷的mRNA的LNPs-mRNA疫苗配方在B16黑色素瘤模型中展示了显著的肿瘤减少、延长生存期和强大的免疫反应。这种配方增加了肿瘤内CD40+ DCs和多功能OVA特异性CD8+ T细胞,显示了有效传递mRNA疫苗的潜力。此外,携带OX40L mRNA的LNP显著减少了小鼠中H22肿瘤的大小。这表明LNPs可以是用于肝癌免疫疗法中系统传递mRNA疫苗的有价值工具。它们也可以作为传递mRNA疫苗的支架。Qiu等人对包裹在LNPs中的mRNA疫苗进行了系统评估。这种疫苗编码HPV的E7蛋白(HPV mRNA-LNP)。接种HPV mRNA-LNP疫苗激活了整体和HPV特异性CD8+ T细胞。此外,它通过肿瘤微环境中的不同IFN反应指导了CD8+ T细胞的功能承诺。与ICIs结合使用时,HPV mRNA-LNP疫苗进一步增强了HPV特异性CD8+ T细胞,同时保持了它们的抗肿瘤功能,导致肿瘤消退增强。这项研究突出了这种方法在癌症免疫疗法中的潜力。
包含甘露糖作为肿瘤特异性mRNA的靶向传递配体的改良LCP-NPs在正位模型中对三阴性乳腺癌(TNBC)展示了强大的CTL反应,通过与抗CTLA-4抗体共传递进一步增强。这突出了LCP系统用于靶向传递肿瘤特异性mRNA的潜力。此外,LCP-NPs被用作由RNAi基于的免疫治疗剂组成的纳米配方的载体。这种方法导致增强的T细胞反应,减少肿瘤生长,并降低转移。这些发现展示了使用LCP作为载体开发mRNA疫苗和其他基于RNA的免疫疗法的潜力。
脂质微粒(LMPs)最近作为传递mRNA疫苗的替代平台出现。像LNPs一样,LMPs可以有效地传递mRNA。它们在与免疫佐剂结合使用时显示出预防癌症的前景,确立了它们作为高效mRNA疫苗传递系统的价值。
这项研究强调了LNPs作为癌症免疫疗法框架中有前景的mRNA疫苗载体的潜力。
mRNA疫苗面临的一个重大障碍是它们易受降解。为了保护mRNA免受降解,设计了新的脂质体介导的传递方法。最近,一种通过将精蛋白和mRNA封装到1,2-二油酰-3-三甲基铵丙烷/胆固醇/1,2-二硬脂酰-sn-甘油-3-磷酸乙醇胺-聚乙二醇(DOTAP/chol/DSPE-PEG)阳离子脂质体中的mRNA疫苗被研究。这种配方展示了增强的摄取效率,并成功刺激了DC成熟,导致对特定肿瘤的显著抗肿瘤免疫反应。此外,由DOTAP、胆固醇缀合阳离子肽DP7、载体佐剂和mRNA组成的脂质体有效地抑制了肿瘤生长,同时增强了淋巴细胞反应。此外,合成了称为甘露糖-胆甾-缀合物(MPn-CHs)的靶向DC的脂质体,并用于配制靶向DC的脂质体(MPn-LPs),增强了mRNA表达并展示了靶向DC的mRNA疫苗的显著潜力。基于脂质体的mRNA疫苗LPX-mLMP2通过将mLMP2封装在DOTAP中合成。LPX-mLMP2展示了APCs对其的印象深刻的抗原摄取,导致有效的LMP2表达。疫苗在脾脏中积累,促进了DC成熟,并在体内引发了针对抗原的T细胞反应,最终在三次疫苗接种后显著减少了表达LMP2的肿瘤模型中的肿瘤生长。在另一种方法中,开发了修饰甘露糖的脂质体以传递总肿瘤mRNA,导致减少转移和黑色素瘤特异性CTL的产生。
最近的研究调查了阳离子脂质体用于mRNA疫苗传递,导致增强的DC成熟、肿瘤生长抑制和特异性免疫反应。
脂质体复合物(Lipoplexes)是阳离子脂质体非病毒合成脂质载体,广泛研究用于个性化癌症疫苗的临床应用。脂质体复合物用于第二代RNA疫苗。从实验室进展到临床使用的第一个产品是设计用于静脉给药的疫苗。它包括四种RNA脂质体药物产品,每个都编码黑色素瘤免疫治疗的常见肿瘤抗原。
I/II期临床试验正在评估针对非突变型癌症相关抗原(CAAs)的RNA-脂质体复合物疫苗,这些抗原包括MAGE-A3、酪氨酸酶、纽约食管鳞状细胞癌1和带有tensin同源性的跨膜磷酸酶,作为单一疗法或与抗PD1抗体联合治疗。这种疫苗能在经预处理、检查点抑制剂经验丰富的IIIB-C期和IV期黑色素瘤患者中引起持久的客观反应。值得注意的是,部分反应的患者表现出强烈的多表位CD4+/CD8+ T细胞免疫的诱导。
由聚乙二醇胆固醇(PEG-chol)和N-十六烷基-N,N-二甲基十六烷-1-溴化铵组成的mRNA脂质体复合物疫苗可以增强细胞内的蛋白表达。当作为免疫接种给小鼠时,这种疫苗增加了肺和脾脏中的蛋白表达,导致抗原特异性IgG1水平升高。尽管这些发现是有希望的,但需要进一步的研究和测试来评估这种疫苗用于人类潜在使用的安全性和有效性。
3.3.聚合物纳米系统用于mRNA疫苗传递
聚合物NPs在促进mRNA传递方面发挥着至关重要的作用,作为支架。在最近的一项综述中,概述了基于聚合物的系统的mRNA传递的关键组成部分,特别强调了分子设计标准、稳定性和生物分布等因素。
聚(β-氨基酯)(PBAE)聚合物可以与mRNA形成复合物。这些聚合物具有生物可降解性的优势,并且能够与水溶液中的带负电荷的mRNA自发自组装。它们具有独特的特性,包括高缓冲能力有助于内质网逃逸,正电荷有助于有效结合mRNA,以及能够水解降解为无毒副产品。最近,Kim等人开发了一种使用PBAE的冻干优化的mRNA疫苗传递载体。这项研究表明,通过胃肠道给药这种疫苗可以有效地传递并刺激免疫系统,导致诱导抗原特异性的细胞和体液免疫。
李等人开发了氟烷基化聚乙烯亚胺PEI(F-PEI)用于mRNA传递。F-PEI增强了细胞内mRNA传递并激活了TLR4介导的信号传导。此外,该团队使用了由F-PEI和编码肿瘤抗原的mRNA组成的自组装纳米疫苗。这些纳米疫苗诱导了DC成熟,实现了有效的抗原呈递,并随后触发了强大的抗肿瘤免疫反应。此外,基于F-PEI的mRNAOVA癌症疫苗在延迟已建立的B16-OVA黑色素瘤生长方面证明了其有效性。与ICIs结合使用时,基于F-PEI的MC38新抗原mRNA癌症疫苗成功抑制了已建立的MC38结肠癌并预防了肿瘤复发。因此,这些疫苗与ICIs的联合使用可能代表了一种增强对癌症的免疫反应并改善治疗结果的潜在策略。未来的临床试验需要验证这些方法在临床设置中的安全性和有效性。
为了mRNA传递,开发了一种新型自组装聚合物胶束,使用改性的PEI共聚物和维生素E琥珀酸酯(PVES)。这种胶束有效地将mRNA传递到各种细胞系,同时造成最小的细胞毒性。在小鼠研究中,使用PVES作为传递载体通过肌肉内给药mRNA疫苗。结果显示出强大的抗体反应,表明PVES是传递mRNA疫苗的安全有效载体。
树状分子是一类具有明确定义的树突状结构和协同多价性的聚合物,可以利用来传递mRNA疫苗。两亲性树状分子载体代表了先进的树状分子,是结合了脂质和聚合物载体特性的混合构建物。脂质组分赋予了与mRNA在其核心内部相互作用和包封的疏水性质。聚合物段提供了修改其表面性质和化学功能的灵活性,以增强靶向和细胞摄取。此外,脂质组分使树状分子能够像基于脂质的载体一样与膜融合,而聚合物段促进了类似聚合物载体的内吞作用,从而减少mRNA传递。这些载体具有精确且稳定的纳米尺寸结构,使它们适合传递mRNA疫苗。树状分子载体有效传递mRNA疫苗依赖于其两亲性质。亲水实体促进RNA结合和疏水性,从而稳定RNA复合物。这一独特的特性使得RNA作为稳定的复合物被包封,促进了其依赖内吞作用的摄取和释放到细胞质中。树状分子的两亲性质在成功的RNA传递中起着至关重要的作用。
基于聚合物水凝胶的疫苗传递系统因其在将疫苗有效地传递到特定生理部位方面的能力而受到研究关注。这些系统以其独特的属性为特征,增强了抗原传递并作为佐剂,增强了抗原诱导的免疫反应。例如,装载mRNA的聚(2-羟乙基甲基丙烯酸)水凝胶成功地传递到DC2.4细胞中。这种方法展示了延长释放和增强转基因表达的潜力,表明其作为提供免疫疗法中mRNA疫苗的替代方法的潜力。此外,聚乙烯亚胺和氧化石墨烯水凝胶被用来开发含有OVA mRNA和佐剂(R848)的纳米疫苗。这种水凝胶实现了mRNA至少30天的持续释放。释放的mRNA保护淋巴结免受降解,并促进了它们的目标传递。此外,实验数据表明,水凝胶显著增强了CD8+ T细胞特异性抗原产生,抑制了肿瘤生长。它还产生了抗原特异性抗体,有效地预防了转移。总的来说,这些发现强调了可注射水凝胶作为mRNA传递有前途方法的潜力。
3.4.基于肽的mRNA疫苗传递
细胞穿透肽(CPPs)因其能够穿越细胞膜并有效地传递核酸而迅速发展。CPPs作为肿瘤免疫疗法药物的潜力巨大。此外,通过结合适当的佐剂和应用如纳米技术等先进技术,可以显著提高mRNA疫苗传递的有效性。为mRNA疫苗传递设计了不同类型的肽(图2)。
图1. mRNA传递系统的示意图,用于高效编码抗原和激活树突状细胞(DCs)
包括封装在DOTAP/胆固醇/DSPE-PEG-精氨酸阳离子脂质体中的mRNA、阳离子DOTAP-胆固醇修饰肽(DP7)脂质体、MPn-CHs脂质体、甘露糖修饰脂质体、mRNA-脂质复合物和脂质复合物+ PEG-胆固醇在内的各种传递系统,有效地将mRNA传递到DCs中。mRNA编码的抗原刺激分泌如TNF-α等细胞因子,导致DC成熟。这些成熟的DCs随后释放IL-2和IL-12,激活CD4+和CD8+细胞,从而抑制肿瘤生长。
Lou等人开发了一种用GALA功能化的mRNA聚合物复合物的方法,展示了高转染效率和无细胞毒性的细胞摄取。用携带OVA mRNA的PPx-GALA配方处理,导致T细胞反应增加和DCs成熟,突出了其作为mRNA疫苗平台的潜力。作为mRNA疫苗传递平台,开发了一种富含精氨酸的CPP变体RALA。这种肽的独特序列和结构组织在作为mRNA疫苗载体的角色中至关重要。它与mRNA形成纳米复合物,能够在酸性pH依赖性方式中破坏膜,促进mRNA内质网逃逸并使其在DCs的细胞质中表达。RALA和修饰的mRNA含有假尿苷和5-甲基胞嘧啶,诱导了针对mRNA编码蛋白的强大CTL反应,突出了它们的优越效力。另一种方法涉及使用阳离子CPP(LAH4-L1)和聚乳酸NPs(PLA-NPs)。产生的纳米复合物通过吞噬作用和有衣小窝被DCs内化,导致大量蛋白表达。CPPs的不同肽序列,连同它们良好的生物相容性和它们所具有的特定目标内化能力,使它们成为一个有前途的替代mRNA传递平台。具体来说,使用大小<200 nm的双极CPP/mRNA复合物已证明能够有效地表达蛋白和细胞摄取。这些发现强调了CPPs作为癌症mRNA传递载体的有效性。
3.5.碳纳米材料用于mRNA疫苗传递
在癌症免疫疗法中,碳纳米材料因其独特的传递抗原和刺激免疫反应的能力而激增,以碳点(CDs)作为杰出的候选材料。Chen等人合成了能够结合mRNA并形成ACDs@mRNA纳米复合物的两亲CDs,显示出对脾脏的最佳目标传递。ACDs@OVA-mRNA配方有效地抑制了E47肿瘤生长,并增强了脾脏和癌症部位的癌症浸润T细胞,为推进肿瘤免疫疗法提供了有希望的结果。此外,荧光CDs被用作疫苗开发中的佐剂,与肿瘤蛋白抗原OVA结合。这种方法导致针对抗原的强大的细胞介导的免疫反应,成功地抑制了黑色素瘤癌症生长。这些研究强调了CDs作为疫苗接种中多功能工具的潜力。它们为改善包括癌症在内的各种医疗环境中的治疗结果提供了激动人心的机会。
图2. 肽介导的mRNA疫苗传递及免疫反应
携带OVA mRNA、RALA、含有假尿嘧啶和5-甲基胞嘧啶修饰的mRNA、阳离子CPP LAH4-L1、PLA-NPs、环肽纳米管(cPNTs)、两性CPP/mRNA复合物以及与海藻酸钠结合的SA@DOTAP-mRNA的聚阳离子肽GALA配方,有效地将mRNA传递到DCs中。信使RNA转录本被转化为蛋白质,然后被蛋白酶体(酶)切割成片段。由此产生的肽片段通过内质网运输,并通过MHC I类(MHCI)呈现给CD8细胞,增强T细胞反应。ER,内质网;TCR,T细胞受体。
碳纳米管(CNTs)因其固有特性,包括惰性、非免疫原性、无毒性和高稳定性,已成为疫苗开发的有希望的传递平台。CNTs的一个关键优势是它们能够有效地运输特定的抗原目标并促进它们向免疫系统的呈递。它们独特的结构允许同时有效附着多种抗原,在其表面产生多种抗原。然而,使用尺寸大于100纳米的碳纳米材料作为mRNA疫苗传递的支架存在几个挑战。这些挑战源于诸如不溶解性、形态不均匀、较大尺寸(小于100纳米)和潜在的肺部毒性等因素。这些考虑使得CNTs在mRNA疫苗传递中的实际应用更加复杂,尽管它们具有明显的优势。研究人员必须解决这些挑战,以充分发挥CNTs在基于mRNA的疫苗领域的全部潜力。
3.6.病毒载体介导的mRNA疫苗传递
病毒载体疫苗属于使用携带编码特定抗原的外来基因的遗传改变病毒颗粒的疫苗类别。在癌症疫苗开发领域,工程化的病毒载体、自复制RNA(小干扰RNA)病毒载体和重组病毒载体已成为有前景的溶瘤病毒(OVs)。这些OVs具有携带单链RNAs、特异性靶向肿瘤细胞并启动适应性免疫的能力。这种新方法在癌症疫苗和疗法的开发中具有巨大潜力。
TroVax()是一种改良的病毒载体疫苗,表达5T4癌症相关抗原。在I/II期人体试验中,它被耐受,没有不良反应,并在前列腺癌、肾细胞癌和结肠癌中诱发了特异性抗体反应。在随后的II期人体试验中,TroVax与化疗药物或ICIs结合使用,增强了抗原交叉呈递和免疫抑制细胞的清除。值得注意的是,与TroVax和同时低剂量IL-2的治疗在肾癌和结肠癌患者的存活率上表现出改善。
图3. 病毒载体介导的mRNA疫苗传递及其对免疫反应的影响的示意图
溶瘤病毒、重组病毒和自复制病毒被设计用于高效地将mRNA疫苗传递到肿瘤部位。这触发了包括先天/适应性免疫机制在内的强大抗肿瘤反应,导致激活凋亡途径、抑制血管生成,以及随后的肿瘤溶解和退行。
一项临床前研究展示了携带与前列腺癌相关的mRNA编码的六跨膜上皮抗原前列腺1的病毒载体平台的有效性。该研究观察到对抗原具有强大、持久的CD8+ T细胞反应,特别是在雄性BALB/c和C57BL/6小鼠株中。令人惊讶的是,尽管疫苗具有高度免疫原性,但其在小鼠PC模型中的保护效率相对较低,无论是可移植的还是自发的。然而,ChAdOx1-MVA疫苗和PD-1抗体组合在临床环境中显示出有希望的抗肿瘤反应。尽管如此,使用病毒载体也存在显著的限制,包括病毒基因组整合到宿主基因组以及宿主排斥的可能性,这可能导致细胞毒性和免疫原性。这些缺点促使人们探索癌症免疫疗法中传递mRNA疫苗的替代策略。
4.结论和未来方向
mRNA疫苗已被公认为一种强大的癌症治疗方法,提供了独特且耐受良好的治疗选择,已在临床前研究中证明有效。这些疫苗可以诱导多克隆免疫反应,并促进保护性抗体的产生,这是通过直接mRNA给药或将其引入DCs实现的。mRNA疫苗的优势在于它们能够编码多种蛋白质,从而克服与特定HLA分子相关的限制。COVID-19 mRNA疫苗的成功激发了人们对其在癌症治疗中潜力的强烈研究努力。
基于mRNA的癌症疫苗提供了编码多种蛋白质、引发强大免疫反应以及促进快速开发和生产的多种优势。然而,一个持续的挑战在于实现有效的mRNA疫苗传递,专门针对肿瘤细胞,这需要进一步的研究和开发。临床前试验已经证明了针对不同类型肿瘤的各种mRNA疫苗的有效性。临床研究集中在针对CAAs的有效mRNA疫苗上。在临床环境中,涉及分离患者自己的DCs并用编码CAAs的mRNA转染的技术正在被研究。基于mRNA的癌症治疗疫苗领域正在迅速发展,有几个领域需要进一步探索和发展。
通过各种传递平台实现mRNA疫苗的有效性,同时最小化不良反应。LNPs可以封装和保护mRNA,促进细胞摄取,并有效地转化为抗原。病毒载体也作为有效的mRNA疫苗载体,但需要解决与安全性、预先存在的免疫力和有限的货物容量相关的挑战。基于肽的传递平台有望增强mRNA疫苗传递和抗原表达。此外,纳米材料如CDs、CNTs和可注射水凝胶已被用于临床前和临床免疫疗法中的mRNA传递。其他技术,包括树状分子和离子电渗,正在被探索。总体而言,癌症免疫疗法中的mRNA疫苗显示出巨大的希望,正在进行的研究努力旨在优化它们的传递、改善免疫反应,并在临床环境中评估它们的有效性。
未来的研究应该集中在改进和优化传递系统,如LNPs、病毒载体和基于肽的平台。这些努力应该旨在提高它们的效率、稳定性、安全性和可扩展性。此外,识别和选择最佳的肿瘤特异性抗原对于提高mRNA疫苗的有效性至关重要。对CAAs和新抗原的额外研究可以导致针对个体肿瘤的有效靶向的个性化疫苗,提高它们的特异性和治疗潜力。探索将mRNA疫苗与ICIs或细胞免疫疗法相结合的协同方案,有望增强抗肿瘤免疫反应。这些联合策略可以克服免疫抑制的TMEs并改善总体治疗结果。进行稳健的人类试验以评估癌症治疗中mRNA疫苗的安全性、有效性和长期效益也是必要的。扩大临床研究的范围并探索不同类型的癌症将为mRNA疫苗的医疗效用提供宝贵的见解,并指导它们未来的发展。
为大规模疫苗接种建立成本效益的mRNA疫苗是具有挑战性的。应努力优化制造技术,降低生产成本,并确保一致的质量和批次间可重复性。对mRNA疫苗的免疫原性和潜在不良反应进行深入调查是必要的,以确保它们的安全性并最小化任何意外的免疫反应。了解mRNA疫苗引起的长期效应和免疫记忆对于它们在临床上的成功实施至关重要。开发稳健的生物标记和标准化的检测方法对于监测mRNA疫苗接种后的免疫反应至关重要,这对于评估疫苗效力、预测患者结果和指导治疗策略至关重要。将免疫监测方法整合到临床试验中可以为优化疫苗设计和剂量方案提供有价值的数据。通过解决这些问题,癌症免疫疗法中的基于mRNA的疫苗领域可以继续发展,为患者提供创新和有效的治疗选择,并有可能改变癌症治疗的格局。
信使RNA疫苗
2023-12-08
与传统疫苗相比,mRNA疫苗因具有高效、快速开发、安全给药和低成本制造等优点,已成为一种有前景的疫苗形式。自20世纪90年代以来,研究人员在临床前模型中连续尝试使用mRNA产生靶蛋白并开发针对传染病的mRNA疫苗。目前,mRNA疫苗在多种疾病的临床前和临床研究中显示出巨大潜力,如自身免疫性脑脊髓炎、肿瘤、寨卡病毒病、甲型流感、冠状病毒病、狂犬病等传染病。mRNA疫苗通常由三部分组成:指导抗原产生的合成RNA分子、mRNA递送材料和佐剂成分。佐剂通常是疫苗中除了非活性抗原之外的额外免疫刺激物,负责激活先天免疫,并提供必要的“帮助”,以提高适应性反应的强度和质量,从而对特定病原体提供最大程度的保护。佐剂可以影响mRNA疫苗的免疫反应类型,并决定是否提高中和抗体水平或增强抗原特异性细胞毒性。因此,加深mRNA疫苗中佐剂的理解至关重要,可以为设计下一代mRNA疫苗提供有价值的见解。基于mRNA固有特性的佐剂佐剂通常是一种免疫刺激剂,通过与先天免疫的PRRs结合引起免疫应答,从而增强疫苗的效力。众所周知,外源RNA分子在哺乳动物细胞中具有诱导免疫反应的潜力。在mRNA疫苗中用于表达抗原的外源性核苷未修饰mRNA通过触发先天免疫信号具有内在的佐剂活性。聚尿嘧啶(U)和钝端含有5'三磷酸的短双链RNA(dsRNA)可以作为mRNA疫苗的佐剂,通过TLR3和RIG-1信号通路增强免疫应答,而不阻碍抗原表达。TLRs和RIG-I信号的激活可诱导产生促炎细胞因子,如肿瘤相关因子-α(TNF-α)、白细胞介素6(IL-6)、IL-12、IL-1β和IFNα/β,这一方面增强了mRNA疫苗所需的保护性免疫,但另一方面可能导致过度炎症。图1 佐剂激活mRNA疫苗中的信号Kariko等人的开创性工作发现没有核苷修饰的RNA分子激活了TLR或RIG-I信号,从而引起抗病毒样免疫反应,这可能损害RNA翻译并导致RNA降解。这种免疫激活可以通过核苷修饰的mRNA来避免,如假尿嘧啶,已广泛用于mRNA疫苗。最近的一项研究表明,辉瑞biontech公司的BNT162b2 mRNA疫苗中修饰的mRNA可能被黑色素瘤分化相关蛋白5(MDA-5)识别,从而触发IFNα的产生,最终促进抗原特异性T细胞和抗体的反应。在大多数情况下,宿主干扰素反应抑制mRNA翻译并诱导RNA降解,但在其他一些情况下,促进mRNA疫苗诱导的抗原特异性反应。有必要进一步研究其潜在机制。总的来说,mRNA疫苗中的RNA分子可以通过内在的免疫刺激特性具有佐剂活性,在设计mRNA疫苗时应该考虑此问题。除了核糖核苷酸序列固有的自佐剂特性外,mRNA还可用于产生功能性免疫蛋白,如细胞因子和抗体,以促进抗原呈递,提高疫苗效力。抗原呈递细胞(APCs)对疫苗学至关重要,但如何有效地将抗原递送到APCs一直是一个挑战。Fc受体在APCs上广泛表达,以识别和结合抗体的Fc部分。因此,将抗原与Fc部分融合是一种将抗原传递到APCs的有效方法。最近有报道称,在K18启动子驱动的人血管紧张素转换酶2(K18-ACE2)转基因小鼠模型中,一种编码SARS-CoV-2人Fc共轭受体结合域(RBD-hFc)的mRNA疫苗可有效预防SARSCoV-2感染。在另一项研究中,利用mRNA编码的组成型活性STING突变体作为佐剂,扩增mRNA疫苗诱导的抗原特异性CD8+T细胞反应,抑制TC-1肿瘤生长。此外,通过mRNA编码免疫刺激分子和免疫检查点阻断抗体来恢复肿瘤微环境的免疫适应性也在mRNA癌症疫苗中进行了测试。例如,TriMix由编码CD40L、CD70和构成活性形式的TLR4的三个mRNA分子组成,目前正在临床试验中作为树突状细胞(DCs)疫苗的mRNA治疗来预防黑色素瘤的进展。mRNA编码双特异性T细胞接合器(BiTE)是消除晚期肿瘤的另一种有希望的治疗方法,它编码一种融合蛋白,在一个片段抗原结合臂上靶向CD3的抗体,在另一个片段抗原结合臂上靶向肿瘤抗原,以诱导持续的内源性双特异性T细胞接合抗体的合成。因此,利用mRNA与特异性功能性免疫蛋白(如STING蛋白、抗CD3抗体)作为佐剂共同产生抗原,是提高mRNA疫苗效力的可行途径。对于肿瘤免疫治疗中的mRNA疫苗,引导mRNA产生抗程序性细胞死亡1(PD-1)抗体等免疫调节剂也可能有助于增强mRNA疫苗的效力,值得进一步研究。基于给药系统成分的佐剂由于生物大分子和负电荷等理化特性,裸mRNA不能有效进入细胞,因此,递送工具是促进mRNA转移到细胞质中进行蛋白质翻译所必需的。虽然有许多方法可以促进mRNA进入细胞质,但脂质纳米颗粒(LNP)最近成为该领域的最佳递送材料。LNP通常由阳离子脂质组成,阳离子脂质与带负电荷的mRNA和结构“辅助”脂质(如胆固醇、磷脂或聚乙二醇偶联脂质)形成复合物。LNP系统中的一些脂质成分具有免疫刺激特性,可以作为mRNA疫苗的佐剂。具体来说,脂质包被的磷酸钙纳米颗粒可以作为人工纳米级Ca2+库,通过在细胞质中提供额外的Ca2+,上调CD80和CD86等共刺激分子,从而促进DC成熟。此外,阳离子脂质成分可能对LNP的佐剂活性至关重要。基于可电离阳离子脂质DLinDMA的LNP具有免疫刺激作用,可作为核苷修饰mRNA疫苗的佐剂,引发具有丰富中和抗体的强效TFH细胞反应和GCB细胞反应。此外,阳离子脂质样物质C1可以将mRNA传递到细胞内空间,促进IL-1β、IL-6、IL-12P70等炎性细胞因子的释放,并通过TLR4信号通路上调共刺激分子的表达。脂质C12-TLRa(由环氧化物C12和含胺TLR7/8激动剂组成)可促进mRNA疫苗递送和TLR应答,同时诱导高水平的中和抗体。另一种用于mRNA疫苗的可电离脂质样材料A2-Iso5–2DC18(A2)由不饱和脂质尾部、二氢咪唑连接体和环胺头基团组成,可激活STING信号传导并释放细胞因子,如C-X-C基元趋化因子10(CXCL10)、I型IFN,以增强免疫应答。非核苷酸STING激动剂衍生的氨基脂质SAL12也可以形成LNP以传递mRNA并诱导IFNβ的产生,从而引发针对SARS-Cov-242的有效中和抗体。二甲基二十八烷基铵(DDA)是一种季铵盐脂质,可与mRNA结合并引发先天免疫和Th1细胞应答,因此可作为狂犬病毒mRNA疫苗的有效免疫佐剂。然而,用于mRNA疫苗的脂质材料的固有免疫刺激特性并不总是对疫苗有益。最近的一项研究报道,mRNA疫苗中的脂质成分(DOTMA和DOPE)代替未修饰的mRNA,可促进单核细胞线粒体ROS的产生,激活NLRP3炎性体并释放IL-1β,从而引发人mRNA疫苗的炎症副反应。这些研究强调了脂质成分独立免疫刺激作用的重要性。因此,筛选合适、可行、具有适当免疫激活功能的脂质成分,对合理设计和开发具有重要意义。表1 mRNA疫苗中具有自佐剂活性的脂质和类脂质物质此外,佐剂的物理性质可能影响其免疫活性。最近,一项研究报告称,在针对呼吸道病毒的疫苗配方中,甘露聚糖的颗粒形式而不是可溶性形式可以作为佐剂。此外,一种显示甘露聚糖的模拟病原体的中空纳米颗粒可以激活DC中的Dectin-2和TLR4受体,并诱导Th17细胞分化以达到抗肿瘤反应。因此,在研制疫苗时还应考虑佐剂的物理特性,如大小和颗粒形态。考虑到许多mRNA疫苗也像LNP系统一样基于纳米递送,也需考虑纳米粒子的物理性质对疫苗效力的影响以及潜在的不良炎症副作用。基于附加免疫刺激剂的佐剂mRNA疫苗中使用的佐剂也可以是免疫刺激剂,而不是脂质成分或mRNA编码的免疫蛋白。富含精氨酸的鱼精蛋白肽可以通过与mRNA形成复合物在体内凝聚并传递mRNA。鱼精蛋白和mRNA形成的复合物可以进一步激活TLR7/8通路,从而引发B细胞和T细胞依赖性应答,对抗甲型流感或肿瘤。含有蛋白蛋白的mRNA疫苗递送平台已在治疗黑色素瘤、前列腺癌和非小细胞肺癌的临床试验中得到评估。另一种肽,胆固醇修饰阳离子肽DP7(VQWRIRVAVIRK),可以激活TLR2-MyD88-IKK-NF-κB通路,改善新抗原疫苗引起的免疫应答,从而作为个体化癌症疫苗的有效佐剂。mRNA疫苗中含有DP7的脂体可有效刺激DC成熟,产生CD103+DC(抗原呈递的主要DC亚型),并分泌促炎细胞因子如TNFα、IL-6、IL-12p70以促进mRNA疫苗的效果。棕榈酸修饰的TLR7/8激动剂R848(C16-R848)已被证明可促进mRNA传递效率并诱导有效的适应性免疫应答。一种糖脂抗原α-GC可以在APCs上的MHC-I样分子CD1d中呈递以激活自然杀伤T(NKT)细胞,也用于mRNA疫苗中以提高抗肿瘤效果。综上所述,mRNA疫苗中具有佐剂活性的免疫刺激剂是功能性肽、天然免疫受体(如TLR)的配体和天然免疫细胞(如NKT细胞)的激动剂。这些额外的免疫刺激剂通常通过激活TLR信号起作用。寻找独立于TLRs的触发先天免疫通路的激动剂作为mRNA疫苗的佐剂可能值得进一步探索。COVID-19 mRNA疫苗中的佐剂成分2019冠状病毒病大流行加速了mRNA疫苗研究的发展。在抗击SARS-CoV-2的战斗中,基于假尿嘧啶修饰的mRNA和LNP递送系统的Moderna和BioNTech疫苗取得了前所未有的成功。mRNA疫苗的纳米递送系统具有载体和佐剂的双重作用。此外,未经假尿嘧啶修饰的mRNA具有潜在的自辅助活性,可通过激活TLRs信号诱导炎症效应。然而,最近的一项临床试验报告显示,CureVac的未修饰mRNA疫苗(CVnCoV)在III期试验中预防SARS-CoV-2感染的有效性仅为47%,远远弱于mRNA修饰疫苗(mRNA-1273和BNT162b2)。此外,对CVnCoV、mRNA-1273和BNT162b2的免疫剂量和中和水平的研究表明,与其他两种疫苗相比,接种推荐剂量时CVnCoV的中和水平最低。对于三种mRNA疫苗的不同疗效,有几种可能的解释。首先,mRNA的免疫原性对疫苗的效力起着至关重要的作用。CVnCoV使用未修饰的mRNA结构,可能作为TLR等PRRs的配体引起炎症反应。有报道指出,mRNA疫苗诱导的I型IFN应答可能建立一种限制初始抗原表达的暂时状态,这不利于疫苗的效力。其次,CVnCoV的免疫剂量为12μg,mRNA-1273和BNT162b2的免疫剂量分别为30μg和100μg。较低的CVnCoV mRNA剂量也可能削弱疫苗的有效性。mRNA疫苗BNT162b2和mRNA-1273在保护人们免受不同病毒变体的COVID-19感染方面同样有效。虽然这两种疫苗都是基于核苷修饰的mRNA结构和LNP技术,但两种疫苗在设计上仍然存在一些差异。mRNA-1273的给药剂量低于BNT162b2。此外,mRNA-1273使用可电离脂质SM-102来实现稳定的纳米颗粒结构,而BNT162b2选择了另一种名为ALC-0315的脂质。BNT162b2和mRNA-1273的免疫原性一般可归因于核苷修饰mRNA的免疫识别、LNP的佐剂活性和疫苗的制造工艺等因素。BNT162b2仍然激活MDA-5信号通路,诱导I型IFN引发抗原特异性T细胞反应并产生中和抗体。LNP除可作为递送剂外,还可作为高效的佐剂。最近,人们发现LNP诱导的先天免疫反应与可电离脂质成分有关,因为含有mRNA的LNP可以诱导趋化因子如CXCL10、细胞因子IL-1β和IL-6的产生,而没有可电离脂质的空LNP失去了诱导有效抗体反应的能力。因此,LNP的佐剂活性也可能是mRNA疫苗BNT162b2和mRNA-1273具有免疫原性的原因。此外,在疫苗制备过程中,在合成mRNA的体外转录(IVT)过程中会产生dsRNA作为副产物。dsRNA可以通过各种细胞内PRRs传感器诱导有效的炎症反应,这有助于mRNA疫苗的免疫原性。这些发现表明,核苷修饰mRNA结构,通过纯化技术优化抗原编码mRNA的制备工艺,以及筛选具有递送和佐剂活性的合适的可电离脂质将是mRNA疫苗成功的关键。综上所述,mRNA疫苗中的佐剂大致可分为三类:(1)从其核苷酸序列或编码蛋白中具有自佐剂活性的RNA;(2)输送系统的组分,特别是LNP中可电离的阳离子脂质;(3)外源性免疫刺激剂。佐剂可以指导mRNA疫苗的免疫应答类型,因此选择合适的佐剂是设计mRNA疫苗治疗不同疾病的关键。一种理想的mRNA疫苗佐剂应该通过激发所需的强效免疫反应来提高疫苗效力,并帮助克服mRNA的缺点,如低稳定性和翻译效率。LNP系统中可电离的阳离子脂质可以极大地影响mRNA疫苗的效力。筛选具有佐剂活性和低毒性的高效递送剂,进一步研究阳离子脂质组分制备mRNA疫苗具有重要意义。此外,利用mRNA编码特异性功能性免疫蛋白和筛选先天免疫受体激动剂作为适用的佐剂也是mRNA疫苗佐剂开发的有希望的方法。寻找有效的佐剂将促进对mRNA疫苗的更好理解,并有助于开发针对不同疾病的更安全、更有效的mRNA疫苗。文献来源:The advances of adjuvants in mRNA vaccines识别微信二维码,添加生物制品圈小编,符合条件者即可加入生物制品微信群!请注明:姓名+研究方向!版权声明本公众号所有转载文章系出于传递更多信息之目的,且明确注明来源和作者,不希望被转载的媒体或个人可与我们联系(cbplib@163.com),我们将立即进行删除处理。所有文章仅代表作者观点,不代表本站立场。
信使RNA疫苗
2022-12-27
·生物谷
mRNA疫苗的研究现状、临床应用及市场机遇
mRNA疫苗的研究现状、临床应用及市场机遇
2022年mRNA疫苗研究方向具有取得了重要的研究从成果,本文中,小编就对本年度mRNA疫苗的研究现状、临床应用及市场机遇等进行整理,分享给大家!
鉴于其卓越的效力、快速的工程设计、低成本的制造和安全的交付前景,信使核糖核酸(mRNA)疫苗提供了一种有趣的替代传统疫苗技术的方法。几个针对传染病和各种癌症的mRNA疫苗平台在体内和体外都显示了有益的结果。与mRNA的稳定性和免疫原性有关的问题已经解决。目前的mRNA疫苗可以产生强大的免疫反应,而不受接受者的主要组织相容性复合体(MHC)单倍型的限制。鉴于基因疫苗是唯一短暂的遗传信息载体,它们也是安全的。
图片来源:10.1016/j.drudis.2022.103458
自20世纪80年代末以来,疫苗诱导宿主免疫反应的能力一直是疫苗研究和开发的核心原则。疫苗需要多糖类抗原(抗原)来诱导天然免疫并提供对感染的保护,而不是依赖于单一的抗原。通过评估疫苗提供的保护水平来降低疾病严重程度和住院率。近年来,基于合成肽、病毒样颗粒、核酸、重组亚单位蛋白和重组病毒颗粒的疫苗研究取得了进展。
一、mRNA疫苗的发展历史
疫苗接种旨在促进对感染病原体和肿瘤细胞的获得性和获得性免疫,从而保护免受病原体和肿瘤细胞的攻击。疫苗介导的获得性免疫在体内既有体液反应,如抗原特异性抗体的产生,作为清除侵袭性感染的第一道防线,也有淋巴细胞(B和T细胞)对抗原或病原体的更持久的细胞介导免疫反应。早期的人类临床试验显示DNA疫苗的效力很差,但改进的递送技术提高了这些疫苗的效力。尽管DNA疫苗和RNA疫苗都提供在宿主细胞中表达的特定重组蛋白,但对mRNA和DNA的利用存在显著差异。首先,单链(Ss)mRNA不如双链(Ds)DNA稳定,因此容易在体内快速降解。然而,mRNA分子可以在宿主细胞的细胞质中直接翻译成蛋白质,而DNA必须被送到细胞核,转录成mRNA,并运输到细胞质进行翻译。设计反式5¢’-甲基葡萄糖苷三磷酸(M7G)帽类似物(M7G),优化3¢’末端的聚(A)尾,以及引入假尿苷等核苷化学修饰,显著提高了转录效率和基因稳定性。此外,为了改善递送和防止在液体和组织中高活性的核糖核酸酶(RNase)对mRNA的降解,已经将mRNA分子包裹在纳米颗粒(NPs)中。
DNA和RNA疫苗可以诱导体液和细胞免疫反应。新出现的病毒疫苗可以快速而容易地开发和修改,以适应突变和新的变异。因此,在严重急性呼吸综合征冠状病毒2(sars-cov-2)的遗传密码被披露仅2个月后,两种基于SARS-COV-2 mRNA的疫苗,mRNA1273和BNT162b2,都是基于脂质的NP配方、核苷修饰的RNA疫苗,进入临床测试,是第一批获得紧急使用授权(EUA)的SARS-COV-2疫苗。然而,RNA疫苗的有效实施受到了限制,因为它们在体内不稳定,而且容易被免疫系统和细胞核酸酶降解,这些问题要求它们在极低的温度(-80摄氏度)下储存和运输。
最近,人们努力优化配方,提供更好的稳定性和延长保质期,以克服这些限制。传统的基因疫苗编码靶基因,其两侧有5’和3’末端非翻译区(UTRs),此外还利用甲型病毒的自扩增RNA(SARNA)开发了基因疫苗。在这种情况下,所传递的mRNA1-4(nsp1-4)包含来自塞姆利基森林病毒(SFV)、辛德比斯病毒(SIN)或委内瑞拉马脑炎病毒(VEE)的非结构基因1-4(nsP1-4),它可以在转基因的宿主细胞中提供20万倍的RNA扩增。这种方法在100-1000倍的剂量下表现出比常规mRNA法更好的免疫应答
1990年,Wolff 等人建立了直接肌肉注射裸质粒DNA用于骨骼肌转基因的方法。尽管最初在20世纪90年代初进行了测试,但由于担心无处不在的RNA引起的不稳定性,RNA疫苗在当时并没有得到广泛应用。因此,大多数基于核酸的疫苗开发的研究都集中在DNA疫苗上,而不是RNA疫苗上。然而,新技术平台的发展证实了RNA疫苗能够诱导强大的体液和细胞免疫反应,这使得基于RNA的疫苗具有吸引力。
图片来源:10.1016/j.drudis.2022.103458
二、 mRNA药物药理研究进展
与基于DNA质粒的疫苗、病毒载体疫苗和传统的减毒活病毒相比,创新的mRNA疫苗有几个显著的好处。与基于DNA的策略相比,基于mRNA的疗法的优点和缺点都有关,似乎有显著的概念性好处。与DNA不同,mRNA不构成染色体整合的潜在危险。治疗性mRNA只产生短暂的表达,并且由于它的SS性质,它在宿主细胞中迅速降解,从安全的角度来看,这可以被视为疫苗开发和癌症治疗的优势。此外,快速引入目标核苷酸序列突变的能力使RNA疫苗非常灵活,这在大流行条件下或个性化治疗中尤其重要。此外,与基于减毒活病毒和病毒载体的疫苗相比,mRNA疫苗是非传染性的,不能整合到宿主基因组中。
对RNA结构的优化进一步提高了RNA的稳定性和翻译能力。例如,在SARS-CoV-2的背景下,将m7GppNmN帽设计到5‘端的工程提高了RNA的稳定性和翻译效率。此外,Poly(A)尾巴和3¢末端非编码区的修饰已被证明有助于改善mRNAs的稳定性和更好的翻译。针对中东呼吸综合征(MERS)冠状病毒S蛋白的受体结合域(RBD)和SARS-CoV-2全长S蛋白或其RBD,通过掺入富GC序列并用1甲基伪尿嘧啶取代尿苷的方法对靶基因的编码区进行了改造,以增强mRNA的翻译。密码子优化,即罕见的密码子被频繁出现的密码子取代,也将翻译效率提高了许多倍。然而,密码子优化也可能影响mRNA的二级结构、翻译动力学和蛋白质折叠,所有这些都可能反过来影响免疫反应的特异性和严重性。
三、 mRNA疫苗的临床应用
mRNA疫苗在感染性疾病中的应用
到目前为止,针对传染病的预防性和治疗性mRNA疫苗已经取得了重大进展,表现出强大的特异性T细胞和体液免疫反应。例如,基于RNA的方法已被开发用于流感病毒疫苗,引起广泛保护性的先天性免疫反应,以抵御同源和异亚型流感病毒的挑战。此外,信使核糖核酸疫苗已经被设计用于兽医,例如在保护小鼠免受口蹄疫病毒(FMDV)挑战的情况下。使用SARNA载体表达狂犬病病毒糖蛋白在免疫小鼠中触发了免疫反应并提供了保护。在另一种方法中,含有POWV(POWV)PrM和E基因的mRNA被包裹在脂质纳米粒中,不仅能诱导针对POWV毒株的强大体液免疫应答,而且还能诱导针对远亲关系的Langat病毒的体液免疫应答。许多动物模型的临床前研究已经证明了用于传染病的mRNA疫苗的可行性,也进行了有限数量的临床试验。
mRNA疫苗在肿瘤中的应用
以mRNA为基础的癌症疫苗是一种独特的方法,它结合了体内生产抗原的潜力和出色的安全性和制造灵活性。表达mRNA的肿瘤相关抗原(TAA)的接种是mRNA疫苗在肿瘤学中最基本的应用。目前已经进行了许多针对mRNA癌症疫苗的临床前研究,一些候选疫苗已经进行了临床试验。临床试验使用了基于TAA的mRNA疫苗,其中树突状细胞(DC)与编码CD70、CD40配体(CD40L)和组成活性TLR4(TriMix)的mRNA电穿孔。接受联合治疗的III或IV期黑色素瘤患者显示出较高的肿瘤反应发生率。活化的TLR4和共刺激分子CD70和CD40L是DC激活和CD8+T细胞应答启动所必需的。为了进一步改变肿瘤微环境(TME),在使用细胞因子混合物刺激DC和T细胞活性后,肿瘤内给药。对IL-23、IL-36和共刺激阻断(OX40L)等细胞因子基因的体外检测可以克服肿瘤抑制环境,产生强大的系统抗癌免疫。不幸的是,目前使用VRP递送的抗原治疗结直肠肿瘤的临床试验仅限于两种(NCT00529984和NCT01890213),而用于癌症疫苗接种的SarNas大多局限于临床前研究。固有的致病相关分子病原体(PAMPs)是限制SARNA使用的关键因素,因为炎症特征的复杂修改可以防止抗肿瘤药物的反复剂量。
mRNA疫苗治疗癌症的临床和临床前实例
图片来源:10.1016/j.drudis.2022.103458
mRNA疫苗在COVID-19中的应用
创新的基于信使核糖核酸的疫苗显示出巨大的前景。虽然传统疫苗技术,如灭活和活减毒病原体和亚单位疫苗接种,可以针对威胁生命的疾病提供长期保护,但要求以低成本和大规模更快地开发疫苗已成为疫苗面临的重要挑战,特别是在大流行的情况下。2019年冠状病毒(新冠肺炎)大流行要求尽快开发针对SARS-CoV-2的疫苗。目前有23种基于RNA的候选疫苗正在临床开发中。
四、 mRNA市场机遇
最近,对mRNA疫苗的临床前和人体研究都出现了热潮,尤其是因为基于mRNA的新冠肺炎疫苗的成功开发。在过去的两年里,大量的临床前和临床数据证明了基于mRNA的技术的有效性。研究表明,mRNA疫苗可以有效地预防一系列传染病,如流感病毒、埃博拉病毒、寨卡病毒、链球菌和寄生原虫弓形虫。此外,针对各种癌症的mRNA疫苗也已开发出来。到目前为止,大多数传染病疫苗都是为了预防而设计的,而大多数癌症疫苗是为了治疗性的。到目前为止,只有两种FDA批准的预防性癌症疫苗获得批准,并用于预防病毒诱导的癌症:乙肝病毒疫苗和人乳头瘤病毒疫苗。肿瘤可以通过细胞毒性T细胞缩小或根除,而细胞毒性T细胞是大多数癌症疫苗的主要靶点。为了开发更有效的癌症疫苗,可以针对在恶性肿瘤细胞中过度表达的TAA。例如,mRNA癌症疫苗可以设计成针对生长相关因子或抗原,这些因子或抗原是由于体细胞突变而对恶性细胞特异的。
三十年的科学和临床进步,再加上研发mRNA新冠肺炎疫苗的巨大努力,预示着mRNA疗法的未来。如上所述,在临床级别,只需点击几下鼠标,就可以自动、可扩展、无细胞地生成编码任何蛋白质的mRNA。在不久的将来,应该有可能产生模块化的、可扩展的良好制造规范(GMP)级制造单元,这些单元可以在任何GMP级设施中建立,从而消除对冷链运输的需求。在这方面,核糖核酸疗法的冷冻干燥即将到来,并将在很大程度上消除目前新冠肺炎核糖核酸疫苗存在的分配问题。随着新的LNP和非LNP载体的开发以及副作用的改善和容量的增加,复杂的基因和碱基编辑以及重复给药可能成为可行的方法,以培育一种新的酶替代治疗方法。
mRNA药物的未来可能取决于以更高的精确度、更长的持续时间和具有可耐受安全性的慢性给药选择,将这种“生命软件”与人类生理系统的生物“硬件”相匹配。在未来几年,mRNA载体、细胞内载体和体内递送系统的快速发展,加上深入的生物学和临床洞察力和直觉,应该会为许多临床需求未得到满足的患者带来新的希望,这些需求无法通过其他治疗方式轻松解决。
参考文献
1. Eduarde Rohner et al. Unlocking the promise of mRNA therapeutics. Nat Biotechnol. 2022 Nov;40(11):1586-1600. doi: 10.1038/s41587-022-01491-z.
2. Alaa A.A. Aljabali et al. Current state of, prospects for, and obstacles to mRNA vaccine development. Drug Discov Today. 2022 Nov 22;103458. doi: 10.1016/j.drudis.2022.103458.
信使RNA疫苗
分析
对领域进行一次全面的分析。
登录
或

生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用



