预约演示
On Target Laboratories Announces Expanded Indication of CYTALUX® (pafolacianine) injection for Detection of Lung Cancer During Surgery
2022-12-16
临床3期上市批准
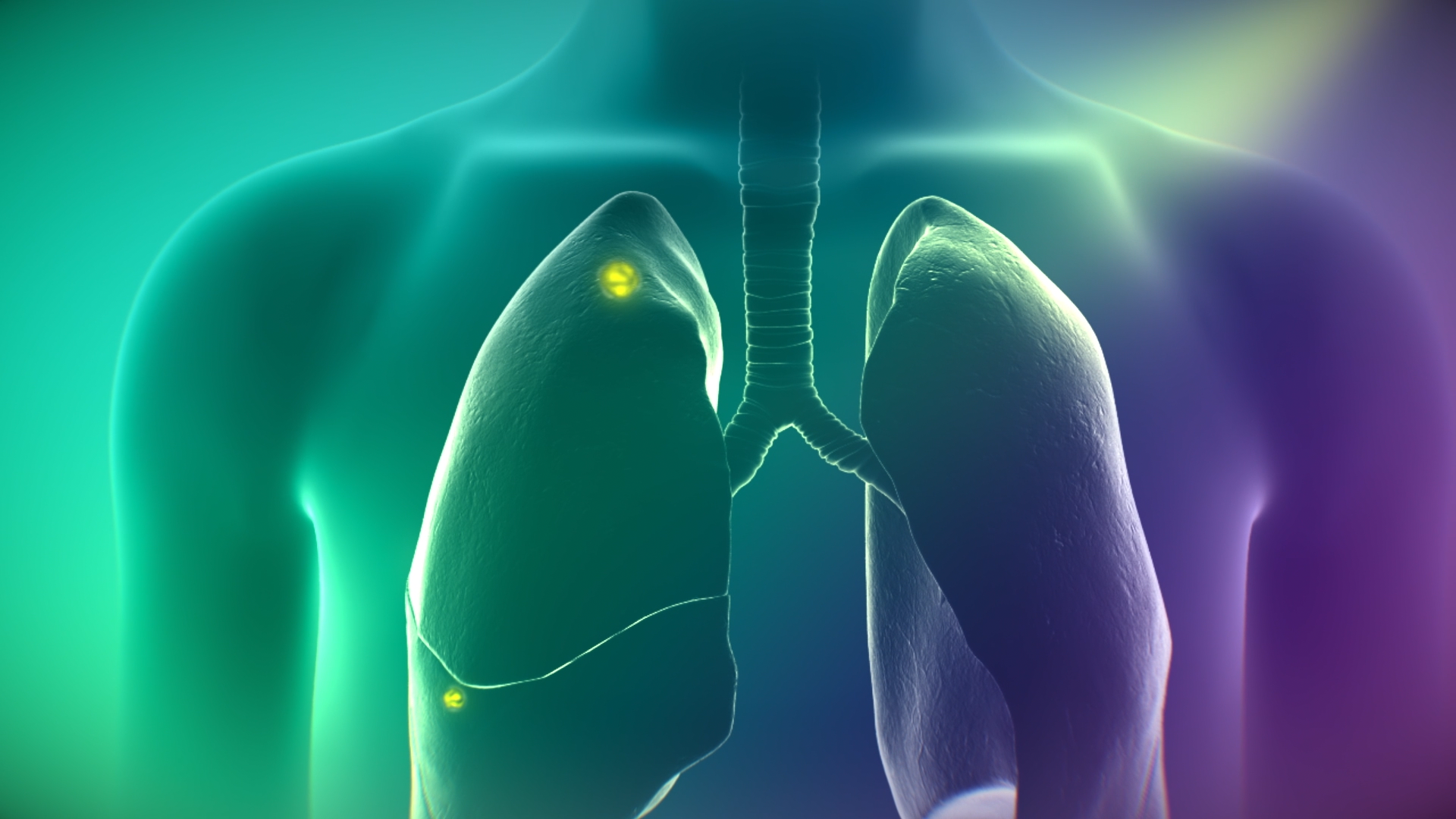
CYTALUX is now approved as the first and only targeted molecular imaging agent that illuminates lung cancer intraoperatively, enabling the detection of more cancer for removal.
CYTALUX is also the first and only targeted molecular imaging agent that illuminates ovarian cancer in real-time during surgery.
WEST LAFAYETTE, Ind., Dec. 16, 2022 /PRNewswire/ -- On Target Laboratories, Inc., a privately-held biotechnology company developing intraoperative molecular imaging agents to target and illuminate cancer during surgery, today announced that the U.S. Food and Drug Administration (FDA) has approved an expanded indication for the use of CYTALUX in lung cancer. CYTALUX is the first and only targeted molecular imaging agent that illuminates lung and ovarian cancer intraoperatively, enabling the detection of more cancer for removal. The new indication will provide surgeons the ability to integrate CYTALUX into their treatment plan for adult patients with known or suspected lung cancer, where it previously was only approved for adults with ovarian cancer.
Continue Reading

Preview
来源: PRNewswire
On Target Lung Cancer Indication Media Kit (Video)
Preview
来源: PRNewswire
Experience the full interactive Multichannel News Release here: https://www.multivu.com/players/English/9123551-on-target-laboratories-expanded-indication-cytalux-injection-lung-cancer-detection-during-surgery/
The label expansion is based on safety and efficacy evidence demonstrated in the ELUCIDATE Trial, a Phase 3, multi-center, single dose, open-label trial that investigated the use of CYTALUX in patients scheduled to undergo thoracic surgery for confirmed or suspected lung cancer.
Lung cancer is the leading cause of cancer deaths, with over 130,000 deaths from the disease in the U.S.1 and nearly 1.8 million globally2 each year. While surgery is a gold-standard treatment, up to 55% of people with lung cancer who undergo surgery with curative intent have a recurrence3. During surgery for lung cancer, some lesions can be difficult for surgeons to visualize, particularly if they are small, beneath the surface of the lung, or a type of lesion called a ground glass opacity, which is becoming increasingly common as the rates of lung cancer screenings rise.
"Today's approval of an expanded indication for CYTALUX marks an important step forward in the treatment landscape for lung cancer and for the more than 220,000 people in the U.S. who receive a lung cancer diagnosis each year," said Chris Barys, President and Chief Executive Officer of On Target Laboratories, Inc. "We are proud to continue pioneering development of targeted intraoperative molecular imaging agents that illuminate cancers intraoperatively to enhance surgeons' ability to see cancer in real time as they operate."
"During the ELUCIDATE Trial, CYTALUX proved to be a valuable surgical tool with its ability to localize lung lesions that may have otherwise been missed," said Dr. Linda Martin, Chief of Thoracic Surgery, University of Virginia School of Medicine. "CYTALUX has potential to become standard of care in thoracic surgery because of the safety and efficacy demonstrated in the trial. It is an important tool for surgeons to consider for patients."
CYTALUX was approved in November 2021 for use in adult patients with ovarian cancer as an adjunct for intraoperative identification of malignant lesions. CYTALUX is the first targeted molecular imaging agent that illuminates ovarian and lung cancer intraoperatively, enabling the detection of more cancer for removal. CYTALUX, administered by standard IV in as little as one hour before surgery, binds to folate receptors that are overexpressed in certain cancers4,5 and illuminates intraoperatively under near-infrared light.
About the ELUCIDATE Trial
The ELUCIDATE (
Enabling
LUng
Cancer
IDentification Using Fol
ATE Receptor Targeting) trial was a Phase 3, multi-center, single dose, open label trial led by Principal Investigator, Sunil Singhal, MD, the William Maul Measey Professor in Surgical Research and director of the Center for Precision Surgery at Penn Medicine and included 12 sites across the United States. The study (NCT04241315) investigated the use of CYTALUX (pafolacianine) injection in patients scheduled to undergo thoracic surgery for confirmed or suspected lung cancer.
About Intraoperative Molecular Imaging
To date, there have been limited ways for surgeons to confidently assess the location and full extent of cancerous tissue while operating. Intraoperative Molecular Imaging (IMI) is an emerging category of technology for surgical oncology in which targeted imaging agents are injected into patients to highlight cancer cells making them visible during surgery.
About On Target Laboratories, Inc.
On Target Laboratories discovers and develops targeted intraoperative molecular imaging agents to illuminate cancer during surgery. Their molecular imaging technology, based on the pioneering work of Philip S. Low, PhD, Purdue University's Presidential Scholar for Drug Discovery and the Ralph C. Corley Distinguished Professor of Chemistry, is comprised of a near-infrared dye and a targeting molecule, or ligand, that binds to receptors overexpressed on cancer cells. The imaging agents illuminate the cancerous tissue, which may enable surgeons to detect more cancer that otherwise may have been left behind.
CYTALUX, the Company's first product, received FDA approval for ovarian cancer in November 2021 and lung cancer in December 2022. CYTALUX targets the folate receptors commonly found on many cancers, binds to the cancerous tissue, and illuminates under near-infrared light. A single dose of the agent is administered via intravenous infusion prior to surgery and assists surgeons in visually identifying additional malignant tissue to be removed during the operation. For more information visit www.ontargetlabs.com and www.cytalux.com.
What is CYTALUX?
CYTALUX is an FDA approved prescription medication that is given prior to surgery to adult patients who have ovarian cancer or known or suspected cancer in the lung. It helps surgeons visualize ovarian and lung cancer lesions during surgery.
Important Safety Information
Infusion-Related Reactions
Adverse reactions including nausea, vomiting, abdominal pain, flushing, allergic reaction, elevation in blood pressure, indigestion, and chest discomfort were reported during the administration of CYTALUX. Your doctor may treat you with antihistamines and/or anti-nausea medication.
Risk of Misinterpretation
Errors may occur with the use of CYTALUX. Sometimes cells may light up even if they are not cancerous or those that are cancerous may not light up. Also, non-cancerous cells from other areas may light up, such as areas of the bowel, kidneys, lymph nodes, lungs, and inflamed tissue.
Pregnancy
CYTALUX may cause fetal harm when administered to a pregnant woman. There are no available human data to evaluate for a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. Contact your healthcare provider with a known or suspected pregnancy.
Folate Supplementation Usage
Folic acid may reduce the detection of cancerous tissue with CYTALUX. Patients should stop taking folate, folic acid, or folate-containing supplements 48 hours before administration of CYTALUX.
Adverse Reactions
The most common side effects of CYTALUX reported in clinical trials were nausea (13%), vomiting (5%), abdominal pain (2%), flushing (2%), other infusion-related reactions (2%), allergic reaction (2%), elevation in blood pressure (1%), indigestion (1%), and chest discomfort (1%) during administration.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of CYTALUX. For more information, ask your healthcare provider.
Call your doctor for medical advice about side effects. You may report side effects to On Target Laboratories at 1-844-434-9333 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See full Prescribing Information for more details.
Media Contacts
On Target Laboratories, Inc.
Jessica Todd
Phone: 513-325-5706
[email protected]
Danielle Kirsch
Phone: 201-693-3197
[email protected]
References
NIH National Cancer Institute. Cancer Stat Facts: Lung and Bronchus Cancer. Accessed December 2022.
Uramoto H, Tanaka F. Recurrence after surgery in patients with NSCLC. Transl Lung Cancer Res. 2014 Aug;3(4):242-9. doi: 10.3978/j.issn.2218-6751.2013.12.05. PMID: 25806307; PMCID: PMC4367696.
Predina JD, Newton AD, Connolly C, Dunbar A, Baldassari M, Deshpande C, Cantu E 3rd, Stadanlick J, Kularatne SA, Low PS, Singhal S. Identification of a Folate Receptor-Targeted Near-Infrared Molecular Contrast Agent to Localize Pulmonary Adenocarcinomas. Mol Ther. 2018 Feb 7;26(2):390-403. doi: 10.1016/j.ymthe.2017.10.016. Epub 2017 Oct 26. PMID: 29241970; PMCID: PMC5835020.
Kalli KR, Oberg AL, Keeney GL, et al. Folate receptor alpha as a tumor target in epithelial ovarian cancer. Gynecologic Oncology. 2008;108(3):619-626.
SOURCE On Target Laboratories, Inc.
更多内容,请访问原始网站
文中所述内容并不反映新药情报库及其所属公司任何意见及观点,如有版权侵扰或错误之处,请及时联系我们,我们会在24小时内配合处理。
生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。





