预约演示
更新于:2025-12-30

Auckland University
更新于:2025-12-30
概览
标签
肿瘤
神经系统疾病
感染
小分子化药
化学药
ADC
疾病领域得分
一眼洞穿机构专注的疾病领域
暂无数据
技术平台
公司药物应用最多的技术
暂无数据
靶点
公司最常开发的靶点
暂无数据
| 排名前五的药物类型 | 数量 |
|---|---|
| 小分子化药 | 9 |
| 化学药 | 2 |
| ADC | 2 |
| 腺相关病毒基因治疗 | 1 |
| 荧光偶联药物 | 1 |
关联
15
项与 Auckland University 相关的药物靶点 |
作用机制 PRF1 抑制剂 |
非在研适应症- |
最高研发阶段临床前 |
首次获批国家/地区- |
首次获批日期- |
靶点- |
作用机制- |
在研适应症 |
非在研适应症- |
最高研发阶段临床前 |
首次获批国家/地区- |
首次获批日期- |
靶点 |
作用机制 GAD1基因刺激剂 |
在研适应症 |
非在研适应症- |
最高研发阶段临床前 |
首次获批国家/地区- |
首次获批日期- |
645
项与 Auckland University 相关的临床试验ACTRN12625000415404
Assessing pain and acceptability of multi-jet injection in healthy volunteers
开始日期2026-06-01 |
申办/合作机构 |
ACTRN12625001130459
The effectiveness of Topical Micronized Purified Flavonoid Fraction Post Excisional Haemorrhoidectomy on Pain: A Double-Blinded Randomised Controlled Trial in Adult patients.
开始日期2026-02-01 |
申办/合作机构 |
NCT04240301
Global PRoMiSe (Perioperative Recommendations for Medication Safety): A Consensus-Based, Generalizable Approach
The purpose of the proposed research is to create and disseminate expert-based recommendations for perioperative medication safety that are tailored to country income level, using the World Bank's four country income groups: high, upper-middle, lower-middle, and low income. The specific aims of the research are to: 1) Develop consensus-based perioperative medication safety recommendations, 2) Prioritize the recommendations by their level of clinical importance for implementation in high, upper-middle, lower-middle and low income countries, and disseminate the recommendations; and 3) Evaluate the initial adoption of the recommendations in each country income group.
开始日期2026-02-01 |
申办/合作机构 |
100 项与 Auckland University 相关的临床结果
登录后查看更多信息
0 项与 Auckland University 相关的专利(医药)
登录后查看更多信息
28,002
项与 Auckland University 相关的文献(医药)2026-01-02·NEW ZEALAND VETERINARY JOURNAL
Article
作者: Katayama, Y ; Bell, A ; Langley, R ; Nakamura, Y ; Middleditch, M ; Hardcastle, M
AIMS:
First, to determine via whole genome sequencing the sequence of the hld gene that encodes δ-toxin and elements of the accessory gene regulator (agr) locus that encode quorum sensing in four Staphylococcus pseudintermedius isolates from atopic dogs; second, to assess degranulation of mast cells by synthetic δ-toxin in vitro, and by culture filtrate containing δ-toxin from the S. pseudintermedius isolates in canine skin in vivo; and third, to determine whether the genetic region (RNAIII) encoding the δ-toxin gene is upregulated in response to increasing bacterial density (quorum sensing) in the isolates.
METHODS:
Four isolates of S. pseudintermedius were obtained from four dogs with pyoderma and canine atopic dermatitis (cAD). All four isolates were sequenced to compare their genomes and the sequences of the agr and hld elements. Synthetic S. pseudintermedius δ-toxin was applied to a mast cell culture from murine fetal liver cells in vitro. Degranulation was assessed using a β-hexosaminidase assay. Filtered supernatants from cultures of the four S. pseudintermedius isolates were tested by mass spectrometry to detect δ-toxin. These filtrates were then injected into the skin of five normal dogs. The injection sites were biopsied 15 minutes later. Degranulation of canine mast cells was assessed and quantified histologically. To assess up-regulation of the genetic region encoding the δ-toxin gene in response to increasing bacterial density in the four S. pseudintermedius isolates, relative expression of RNAIII was assayed using quantitative PCR after 1, 2, 4, 7 and 8 hours of culture.
RESULTS:
Synthetic S. pseudintermedius δ-toxin caused comparable degranulation of MC/9 cells to δ-toxin of Staphylococcus aureus. Mast cell degranulation was demonstrated in the skin of all five normal dogs following intradermal injection of a purified supernatant that contained S. pseudintermedius δ-toxin. The genetic elements of the δ-toxins were described. As the cell density of cultures of the S. pseudintermedius isolates from atopic dogs increased, RNAIII expression increased relative to the reference gene (gyrB), suggesting that RNAIII expression may be controlled by a quorum-sensing mechanism.
CONCLUSIONS AND CLINICAL RELEVANCE:
S. pseudintermedius isolates from atopic dogs carry genes encoding δ-toxin, a staphylococcal exotoxin that can degranulate murine mast cells in vitro. An agent in filtered S. pseudintermedius culture known to contain δ-toxin causes degranulation of dermal mast cells in vivo and may play a role in the initiation and/or exacerbation of cAD.
2025-12-17·ACS Applied Materials & Interfaces
Ultrasmall Gold Nanoparticles as “Three-in-One” Enzyme-Mimicking Nanocatalysts for Combined Sonodynamic/Catalytic Therapy in Breast Cancer
Article
作者: Chung, Lip Yong ; Lim, Xin Yun ; Kiew, Lik Voon ; Hwang, Dennis W. ; Leo, Bey Fen ; Seema, Jaya ; Teo, Yin Yin ; Lin, Chung-Yin ; Beishenaliev, Adilet ; Chang, Chia-Yu ; Huang, Yuhan ; Chen, Yu-Wen ; Chang, Chia-Ching ; Goh, Sook Jing ; Loke, Yean Leng
The combination of catalytic therapy and sonodynamic therapy (SDT) emerges as a promising approach for the treatment of solid tumors. Smartly designed nanocatalysts can activate tumor-localized catalytic reactions to convert hydrogen peroxide (H2O2) into highly toxic hydroxyl radicals (•OH) while simultaneously generating singlet oxygen (1O2) under ultrasound stimulation to induce massive oxidative bursts in cancer cells. However, inadequate levels of endogenous H2O2 in tumor tissues and poor sonocatalytic performance of existing nanocatalysts remain major challenges for SDT/catalytic therapy. Self-sufficient nanocatalysts that can generate H2O2 within the tumor microenvironment offer a way to continuously power further catalytic reactions, effectively overcoming the limitations of monomodal nanocatalysts. However, such complex nanocatalyst systems with multienzymatic and sonosensitizing properties are often challenging to translate from the bench to the bedside. In this study, we employed ultrasmall gold nanoparticles (usAuNPs) as "three-in-one" multifunctional yet structurally simple nanocatalysts. Under the mildly acidic environment of tumors, usAuNPs were shown to decompose H2O2 into •OH via peroxidase-like activity and self-supply H2O2 by breaking down glucose via glucose oxidase-like activity. Meanwhile, usAuNPs can be activated by ultrasound (1 MHz, 2.0 W/cm2) to generate a significant amount of 1O2 (k = 1.78 × 10-1 min-1), which is 4-7.5-fold greater than other reported gold nanocomposites. In vivo experiments showed that usAuNP-mediated SDT/catalytic therapy can cause significant suppression of breast cancer growth, achieving a tumor growth inhibition of 90% following a single dose of nanoparticles. Importantly, their ultrasmall sizes facilitate tumor-specific accumulation and rapid clearance from the body via renal filtration, achieving enhanced cancer eradication without significant systemic toxicity. Overall, this work spotlights a potential use of usAuNPs as effective and simple sonocatalysts for combined SDT/catalytic therapy.
2025-12-02·Autophagy
Impaired MAPT/tau-secretory lysosomes are linked to cognitive vulnerability in Alzheimer patients
Article
作者: Prasad, Vidya Mangala ; Gandasi, Nikhil R ; Saroja, Sivaprakasam R ; Sharma, Preeti ; Chatterjee, Ananya ; Pallavi, Anuma
MAPT/tau proteins propagate between brain regions in a prion-like manner, driving the onset and progression of dementia in Alzheimer disease (AD). However, the basis for variability in dementia progression among AD patients remains poorly understood. Here, we demonstrate that cognitively resilient AD patients, characterized by reduced MAPT/tau pathology, maintain lysosomal integrity, whereas cognitively vulnerable patients, exhibiting greater MAPT/tau burden, display lysosomal dysfunction. Lysosomes in cognitively vulnerable AD brains contain partially digested, seed-competent MAPT/tau species composed mainly of the amyloidogenic core with degraded peripheral regions. These pathogenic MAPT/tau forms are secreted via lysosomal exocytosis, facilitating MAPT/tau propagation and contributing to cognitive decline. Cognitively vulnerable female AD patients show increased lysosome-mediated MAPT/tau secretion relative to their male counterparts. Our findings suggest that lysosomal dysfunction, marked by altered protein expression, pH dysregulation, and MAPT/tau accumulation, underlies the heterogeneity in dementia severity. Targeting lysosomal exocytosis and the amyloidogenic core of MAPT/tau fibrils offer a promising therapeutic avenue to mitigate MAPT/tau pathology and promote cognitive resilience in AD and related dementias.Abbreviation: AD: Alzheimer disease, LAMP1; lysosomal associated membrane protein 1, NFT: neurofibrillary tangles; MAPT: microtubule associated protein tau; PHF: paired helical filaments; TIRF: total internal reflection fluorescence; TARDBP/TDP-43:TAR DNA binding protein.
136
项与 Auckland University 相关的新闻(医药)2025-12-10
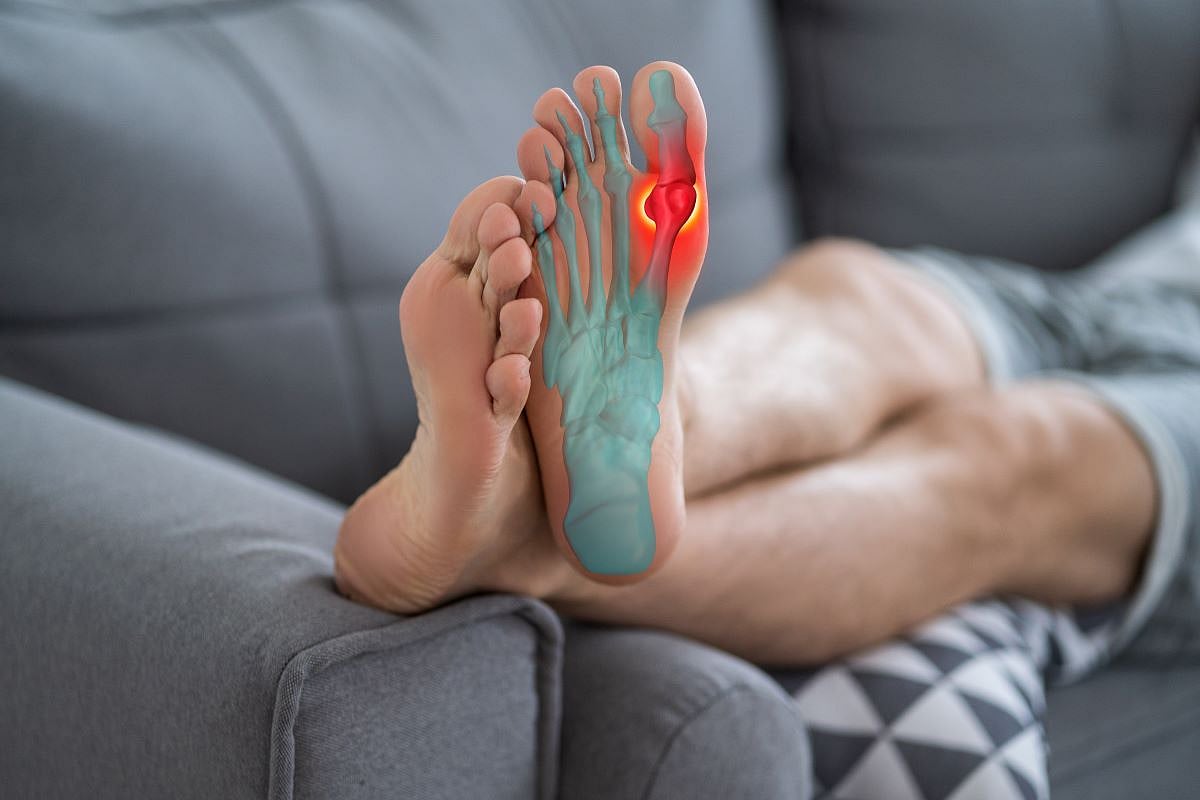
WEDNESDAY, Dec. 10, 2025 — Gout patients shouldn’t trust TikTok videos for advice on managing their condition, a new study says.
Most videos portray gout flares as a personal choice that can be alleviated through a healthy diet and less booze, researchers reported today in the journal Rheumatology Advances in Practice .
The videos fail to focus on effective medical treatments for gout, including drugs that lower uric aid levels and reduce swelling, researchers said.
“TikTok promotes a wide range of information about gout that may be misleading or inconsistent with medical guidelines,” a team led by Samuela Ofanoa , a research fellow at the University of Auckland in New Zealand, concluded.
“The majority of videos highlighted dietary risk factors and management strategies, potentially reinforcing stigma and individual blame,” researchers wrote. “Herbal remedies and dietary supplements, which lack scientific validation, were also common.”
Gout is a painful inflammatory arthritis caused by high levels of uric acid in the blood. Uric acid crystallizes and forms deposits in the joints, causing painful swelling in the hands and feet.
An estimated 41 million people worldwide suffer from gout, with about 7 million new cases every year, researchers said in background notes.
For the new study, researchers analyzed 116 TikTok videos that came up when searching for the term “gout.”
About 45% of videos mentioned risk factors for gout, with 90% blaming the health problem on diet and lifestyle choices, researchers found.
Diet and alcohol are risk factors for gout flares, but genetics, kidney impairment and weight play a significantly greater role, researchers said.
Likewise, 79% of TikTok videos addressed gout management, but 53% of those focused on diet advice.
For example, some videos reported foods to avoid. One patient hospitalized for gout said viewers “can reduce your incidences of gout if you cut back on your salt, your alcohol and your red meat,” researchers said.
Videos also commonly referenced supplements, herbal and home remedies for gout management, promoting products such as “pills made from pure herbs, with no hormones and no side effects,” researchers said.
However, diet has limited long-term effectiveness in treating gout, researchers said.
Only seven of the 116 videos discussed medications to manage gout, and results showed these mainly focused on pain relief options such as steroids or over-the-counter pain relievers like ibuprofen and naproxen.
Just two TikTok videos mentioned using drugs like allopurinol to lower uric acid levels in the bloodstream, even though this is the standard treatment recommended by rheumatologists, researchers said.
"TikTok has great potential as a tool to raise awareness around health issues such as gout and promote information that aligns with clinical guidelines," ‘Ofanoa said.
"In an increasingly digital world, there is a need for more health professionals and organizations to seize the opportunity that social media platforms present, and create content that can counter misinformation and improve understanding about gout in our communities,” ‘Ofanoa continued.
Sources
Oxford University Press, news release, Dec. 10, 2025
Rheumatology Advances in Practice, Dec. 10, 2025
2025-11-23
·汇聚南药
邹秉杰
中国药科大学特聘研究员、博士生导师,药学院药物分析系主任。江苏省杰出青年基金、江苏省“333 人才”、江苏省“六大人才高峰”人才获得者。在 Angew Chem Int Ed, Nucleic Acids Res, Chem Sci, Genome Bio, Biosens Bioelectron 等杂志发表论文 50 余篇。获教育部科技进步一等奖,江苏省医学新技术引进一等奖、二等奖等奖项。担任中国药理学会分析药理学专业委员会常务委员。主要研究方向: 1)核酸与蛋白等生物大分子分析新技术; 2)疾病分子诊断试剂盒研发; 3)单细胞分析新方法。
宋沁馨
中国药科大学药学院药物分析系教授、博士生导师。江苏省 333 工程第三层次培养对象,江苏省青蓝工程中青年学术带头人,中国药理学会分析药理学专委会委员,江苏省药学会药物基因组委员会委员。新西兰皇家科学院、奥克兰大学、维多利亚大学、日本日立中央研究所访问学者,科技部中新科学家交流计划成员。主持国家自然科学基金、江苏省自然科学基金和江苏省社会发展重点项目等纵向课题 20 余项。主编英文、中文专著各 1 部,副主编中文专著 4 部,参编著作 9 部。获教育部科技进步一等奖、江苏省科技进步三等奖、云南省科技进步一等奖、江苏省教育科研成果二等奖、江苏省高校教师教学创新大赛二等奖、南京市优秀论文奖、教育部课程思政教学名师、全国药学专业学位研究生优秀学位论文指导老师、江苏省普通高校优秀毕业论文奖指导老师等奖项和称号。
基于免疫分析法的单细胞蛋白质检测技术研究进展 PPS
邓朝霞,王梦灵,尹王文康,姚春露,张惟杰,王琛,宋沁馨 *,邹秉杰 **
(中国药科大学药学院 药物质量与安全预警教育部重点实验室,江苏 南京 210009)
[ 摘要 ] 单细胞分辨率下解析蛋白质表达,对探究细胞异质性、肿瘤微环境及推进临床精准医学等基础生物学研究具有重要意义。伴随材料化学、核酸化学、蛋白质化学的进步,以及新型检测设备与数据处理系统的迭代升级,单细胞蛋白质检测方法快速发展。其中,基于免疫分析法的单细胞蛋白质检测技术,凭借良好的靶向性和广泛的适用性,成为单细胞蛋白质分析的重要手段。综述了基于免疫分析法的单细胞蛋白质检测技术的原理、优缺点及应用,并对单细胞蛋白质分析技术的发展趋势进行了展望。
细胞是生物体结构与功能的基本单元,除病毒外,几乎所有生物体均由细胞构成。研究机体细胞的功能及其协同机制,对揭示生命本质具有重要意义。尤其在肿瘤研究中,细胞异质性导致肿瘤发生、发展进程及治疗响应存在显著差异 [1]。因此,深入了解肿瘤细胞异质性以及肿瘤微环境中各类细胞的相互作用,是实现癌症精准诊疗的关键。在此背景下,单细胞分析技术应运而生。通过解析单个细胞内功能性分子,该技术能够揭示细胞功能及肿瘤细胞异质性相关机制,为阐明疾病病因、筛选治疗靶点及探索新型治疗模式提供重要依据 [2]。
随着高通量测序技术的进步,多种单细胞基因分析技术相继涌现,包括CEL-seq, MARS-seq, Paired-seq, SMART-seq 和 ScNaUmi-seq 等 [3], 以及用于单细胞空间转录组学分析的 10×Visium[4], Image-seq[5], Slide-tags[6], Slide-seqV2[7], MERFISH和 seqFISH[8] 等。这些技术极大地推动了细胞功能研究,各器官与组织的细胞基因和转录图谱正逐步完善。然而,仅从中心法则上游分子层面解析细胞功能存在局限性,对单细胞内蛋白质等功能大分子的分析,才能更直观地反映细胞的生理状态与功能特性 [9]。因此,单细胞蛋白质分析技术已成为当前研究的重要方向。
目前,单细胞蛋白质分析技术中,除单细胞质谱等少数直接检测方法外,多数依赖基于抗体识别的免疫分析技术。抗体凭借高亲和力和强特异性,能够靶向单细胞内低丰度蛋白质,与传统方法相比,显著降低了检测限,提升了检测灵敏度。通过与流式细胞术、微流控芯片、质谱、纤维荧光成像、拉曼光谱分析等技术联用,免疫分析方法还可实现高通量检测,并获取蛋白质迁移信息,为下游特定表型细胞的分离与分析提供支持。本文将系统综述近年来基于免疫分析法的单细胞蛋白质检测技术的原理、优缺点及其在药理学研究中的应用,并对该领域未来发展趋势进行展望。
1
单细胞蛋白质免疫印迹技术
基于群体细胞的蛋白质印迹法起源于 20 世纪80 年代,现已发展至单细胞水平。该技术通常利用微流控技术分离单个细胞,使其沉降于凝胶中,再经细胞裂解、电泳、转膜、抗体结合及显影等常规蛋白质印迹流程,实现单细胞蛋白质检测。
Liu 等 [10] 开发了液滴印迹法( DropBlot analysis, DropBlot)。该技术通过油包水体系将单个经多聚甲醛固定的细胞封装于微液滴中,利用电转移技术将液滴内细胞裂解物定量转移至聚丙烯酰胺( polyacrylamide, PA)凝胶芯片的微孔内,实现凝胶内单细胞蛋白质免疫印迹( single-cell Western blotting, scWB)。 DropBlot 技术成功检测到保存 6年以上的人源性乳腺癌肿瘤标本中的4种蛋白靶标,且支持在100 ℃高温下孵育1~2小时进行细胞裂解,适用于生物储存库中不同固定条件的细胞样本分析。
然而, scWB 技术仍存在灵敏度不足和检测通量低的问题。为降低检测限, Long 等 [11] 将电泳分离与抗体检测过程解耦,先将蛋白质转移至硝酸纤维素膜,再使用酶标抗体进行检测,有效放大检测信号,实现低丰度靶标的检测。应用该改进方法检测表达增强型绿色荧光蛋白( enhanced green fluorescent protein, EGFP)的 HEK293 T 细胞,阳性检出率达 100%,较传统凝胶内检测方法提高了15.5 个百分点。针对 scWB 重复抗体剥离过程限制多重蛋白检测的问题, Lomeli 等 [12] 开发了基于飞行时间多路离子束成像的单细胞免疫印迹技术( multiplexed ion beam imaging by time-of-flight of single-cell immunoblotting, scIB-MIBI-TOF)。该技术采用金属标记抗体,避免光谱重叠,将单次可检测蛋白种类从约 3 种提升至约 40 种,大幅提高检测通量,并成功检测到 U251-tGFP 细胞中 2 种不同形式的 EGFP。该技术拓展应用于免疫细胞和耐药相关蛋白检测,有望用于预测异质肿瘤的耐药性。 Xu等 [13] 则提出基于荧光-淬灭适配体的单细胞蛋白质印 迹 法( fluorescent-quenching aptamer-based singlecell Western blotting, FAS-WB),设计了一对分别标记荧光基团和淬灭基团的互补适配体。每轮成像由荧光适配体识别靶标,成像后通过杂交互补适配体淬灭荧光信号,实现连续检测。该迭代淬灭策略突破了多重检测限制,省去抗体剥离步骤,避免信号损失;同时,适配体因尺寸小,可更快渗透进入PA 凝胶。 FAS-WB 每个检测周期不到 25 分钟,成本仅为传统抗体探针的 1/1 000,显著提升了检测效率。通过同时检测 Lncap 细胞内癌抗原 125、上皮细胞黏附分子、蛋白酪氨酸激酶 7、人表皮生长因子受体 2( human epidermal growth factor receptor 2, HER2)这 4 种蛋白,验证了该方法的有效性。
尽管上述改进在一定程度上提升了单细胞蛋白质检测的灵敏度和通量,但 scWB 技术仅适用于裂解后的细胞,无法保留细胞的完整性及位置信息,限制了其在临床领域的应用。
2
基于微流控的单细胞免疫检测技术
细胞内多数蛋白质丰度较低,亟需高灵敏度和高特异性的分析方法。近年来,微流控设备与电化学发光、荧光免疫(微珠或固相载体)、侧流免疫层析等免疫检测平台的融合,使微型免疫检测技术成为研究热点。如典型的荧光微流控免疫分析法通过微流控设备将单个细胞捕获至独立微室,利用微室中的荧光条形码免疫磁珠,实现细胞表面蛋白或裂解后胞内蛋白的检测,根据荧光颜色和强度即可解码出蛋白种类与丰度。
Lu 等 [14] 基于钌标记抗体在三丙胺存在下于电极表面氧化发光的原理,开发了电化学发光检测系统,用于活细胞表面蛋白检测。研究发现,经肿瘤坏死因子 α、淋巴素( lymphonectin, LT) α3 和LTα1β2 等活化剂刺激后,人脐静脉内皮细胞的细胞间黏附分子-1 表达显著上调,与预期相符。该方法背景信号低,信噪比高,无需繁琐的细胞分离步骤,避免了分离过程中细胞表面蛋白的改变,但检测蛋白通量仍存在局限。
相对于电化学法,荧光免疫法因检测通量高而备受青睐。 Armbrecht 等 [15] 构建了含 1 026 个微室(单室体积 152 pL)的微流控平台,可同时捕获单细胞与荧光条形码免疫磁珠。细胞裂解后,释放的蛋白与磁珠结合,通过磁珠荧光特征实现蛋白定性定量分析。该团队应用此平台对 MCF-7, SK-BR-3和 HEK-293T 细胞内甘油醛-3- 磷酸脱氢酶、半乳糖凝集素-3 和半乳糖凝集素-3 结合蛋白进行多重免疫测定,结果与高通量 RNA 测序数据一致,检测限低至 1.8×103 个分子。后续研究通过增设物理控制阀 [16] 优化芯片结构,实现全血中循环肿瘤细胞( circulating tumor cell, CTC)的物理分离,在检测乳腺癌单个 CTC 分泌的粒细胞生长刺激因子时,检测限达 1.5 μg·L-1,灵敏度和特异性优异,细胞捕获效率达95%以上,集成化设计显著提升临床样本检测效率。
为实现单细胞蛋白的高灵敏检测, Yu 等 [17] 结合普通荧光显微镜、自制毛细管皮升操作设备及气泡黏附细胞取样技术,开发了单细胞皮升液体操 作 技 术( single-cell picoliter liquid operating technology, scPILOT)。该技术采用量子点标记抗体微阵列,通过显微成像计数单细胞中超低拷贝数蛋白,可在 50 pL 反应单元内检测单细胞中 1 ~ 1 500 个拷贝的超低丰度蛋白,线性与重复性良好。应用该技术检测三阴性乳腺癌细胞中 HER2 蛋白,揭示其表达存在显著细胞间异质性,为信号网络中关键蛋白的验证和定量提供了有力工具。 Lv 等 [18] 设计了近红外光( near-infrared light, NIR)响应微流控芯片,整合尺寸选择性侧流微阵列与金纳米棒预嵌明胶( NIR 响应水凝胶)捕获单元,实现高纯度、高活力单 CTC 的捕获与位点特异性释放。临床检测结果显示,该芯片可从 11 名癌症(乳腺癌、肺癌、结肠癌)患者血液中成功捕获 CTC,而 5 名健康对照样本均呈阴性,验证了其临床应用价值。
总体而言,微流控芯片与免疫分析法结合的单细胞蛋白质检测技术,兼具裂解细胞与完整活细胞检测能力,可覆盖胞内蛋白、膜蛋白及分泌蛋白检测,在临床药理学研究中优势显著。该技术操作简便,可自动化完成单细胞分离与分析,但受荧光通道数量限制,检测靶标通量仍有待提升,且难以提供蛋白质在细胞内的空间分布信息。
3
质谱流式细胞技术
质谱流式细胞术( mass cytometry, MC)是基于质谱原理的单细胞多参数检测技术,通过过渡元素标记抗体,利用抗体和待测样品的免疫结合,最终借助电感耦合等离子体-质谱( inductively coupled plasma-mass spectrometry, ICP-MS)进行检测。该技术最早被称为飞行时间质谱流式细胞术( cytometry by time-of-flight mass spectrometry, CyTOF),目前主要分为悬浮质谱流式细胞术( suspension mass cytometry, SMC)和成像质谱流式细胞术( imaging mass cytometry, IMC)两大类。
Bendall 等 [19] 使用过渡金属同位素标记抗体,将结合抗体-同位素偶联物的细胞以单细胞液滴形式喷入约 5 500 K 的电感耦合氩等离子体中,促使细胞蒸发并产生原子电离,随后利用飞行时间质谱仪对元素离子进行采样和定量分析。该方法可同时测定单个人骨髓单核细胞中的 31 种靶标蛋白,突破了荧光通道限制,为解析健康人造血系统免疫信号提供了全面视角,标志着 SMC 在单细胞生物学研究中的重要突破。为获取空间信息, Giesen 等 [20]将免疫细胞化学与高分辨率激光消融技术联合应用于 CyTOF,开发出 IMC,可在亚细胞分辨率下同时成像 32 种蛋白质及其修饰。利用该技术分析磷酸酪氨酸磷酸酶抑制剂处理的 HMLE 细胞,发现丝裂原活化蛋白激酶( mitogen-activated protein kinase, MAPK)通路和 Janus 激酶-信号转导与转录激活因 子( Janus kinase-signal transducer and activator of transcription, JAK-STAT)通路相关蛋白,如细胞外信号调节激酶(extracellular signal-regulated kinase, ERK) 1 的 T202 和 Y204 位 点、 ERK2 的 T184 和Y186 位点、 p38 丝裂原活化蛋白激酶(p38 mitogenactivated protein kinase, p38MAPK)的 T180 和 Y182位点、 STAT5 的 Y694 位点磷酸化水平显著升高,而核因子-κB 通路、 AMP 活化蛋白激酶通路等非酪氨酸磷酸化位点无明显变化。此外, IMC 应用于人类乳腺癌样本时,能够表征细胞亚群、细胞间相互作用及肿瘤异质性。 Baughn 等 [21] 采用 SMC 方法,对 49 份新诊断或复发/难治性多发性骨髓瘤( multiple myeloma, MM)患者的原发性肿瘤样本进行分析,检测 34 种靶标,鉴定出 13 个表型元簇,绘制出原发性MM首份大规模单细胞蛋白质图谱。 Sheng等 [22]设计高复用 IMC 检测面板,量化 134 名肝癌患者及7 名健康供体样本中的 36 种组织生物标志物,生成562 张单细胞分辨率的高复用组织学图像,首次揭示肝癌肿瘤微环境的多种拓扑功能单元。
尽管 MC 技术显著提升了靶标检测通量并实现了对蛋白位置信息的获取,但其仍存在局限性:其一,检测能力受金属同位素数目的限制,难以达到组学水平的多重检测;其二,金属同位素标记抗体成本高昂,导致检测费用居高不下。
为了克服以上问题,研究者开发了多种质量标签试剂,包括金属螯合聚合物( metal chelating polymer, MCP)、小分子质量标签、聚合物微珠及纳米粒子标签等。 Zhang 等 [23] 通过巯基-马来酰亚胺反应和叠氮-二苯并环辛炔点击反应,在铂/二苯胺复合物的悬垂基团上引入低聚乙二醇(polyethylene glycol, PEG)合成制得新型铼质量标签,解决了传统 MCP 标签仅能结合硬金属离子及易发生聚合物沉淀的问题;该聚合物标签可与商用金属偶联抗体协同用于多参数单细胞免疫检测,拓展了质量标签的应用范围。 Dang 等 [24] 报道了基于纳米金属有机框架( nanometal organic framework, NMOF)的Zr-NMOF 质量标签,直径约 33 nm,在水中分散均匀且稳定性高(储存期超 1 年),信号放大倍数较MCP 标签增加 5 倍,适用于 CyTOF 中的高灵敏和高通量单细胞生物标志物检测,且与其他质量标签兼容性良好。 Chen 等 [25] 开发基于 PEG 功能化的介孔 卟 啉 框 架(mesoporous porphyrinic framework, MPF)用于 SMC 分析,该 MPF 可螯合 Sn, W, Pt, Pd 等 27 种非镧系金属稳定同位素,有效扩展了 CyTOF 的检测通道,克服了传统 MCP 材料中负载金属种类有限的缺点。 Liu 等 [26] 采用聚苯乙烯纳米颗粒这一极易得的经典材料作为金属载体,通过优化细胞染色缓冲液降低非特异性结合,灵敏度较传统 MCP 标签提高 5 倍,为增加检测通道提供了低成本方案。
随着 MC 技术的发展,其与其他技术的联用在临床研究中展现出重要价值。 Wang 等 [27] 将 SMC与基于质谱的无标记单细胞蛋白质组学进行整合,探 究 了 强 直 性 脊 柱 炎( ankylosing spondylitis, AS)的免疫特征。该研究纳入 44 名未经治疗的AS 患者、 14 名经非甾体抗炎药和抗风湿药物治疗的 AS 患者和 31 名健康个体,揭示出 32 种免疫标记物与细胞表型的临床关联,为系统地了解 AS 的发病机制提供了新的见解,有助于进一步开发新的治疗策略。
目前, MC 技术可检测裂解细胞及固定细胞,适用于细胞内各类蛋白质检测,已在医院、高校等科研机构逐步应用。然而,昂贵的仪器设备和较高的分析成本,限制了其更广泛的普及与应用。
4
免疫荧光显微成像技术
免疫荧光常与荧光显微镜联用,以成像方式呈现数据。其优势在于能够以亚细胞分辨率提供细胞位置信息,结合邻近技术,可间接推断蛋白-蛋白相互作用( protein-protein interaction, PPI),直观反映细胞生理过程。免疫荧光主要有 3 种模式:直接使用荧光标记一抗检测靶蛋白;通过荧光标记二抗间接放大一抗信号;新兴的荧光标记核酸法则为抗体添加核酸标签,利用核酸的可编程性和可扩增性,将蛋白质信号转化为核酸信号,显著提升检测灵敏度和检测通量。
Cai 等 [28] 开发的快速多重免疫荧光法,利用特殊的可重复标记染料,突破传统荧光显微镜通道限制,实现 25 种标记物同时成像。通过单像素分析,该方法成功揭示了 WNT/β-连环蛋白通路和 v-akt 鼠胸腺瘤病毒癌基因同源物编码蛋白/哺乳动物雷帕霉素靶蛋白通路中信号蛋白的表达水平与核-质易位现象,解析了激酶的磷酸化活性及其在信号通路中的调控作用。针对染料直标抗体检测低丰度蛋白灵敏度不足的问题, Saka 等 [29] 提出通过交换反应信号放大的免疫染色( immunostaining with signal amplification by exchange reaction, Immuno-SABER)技术,通过 DNA 条形码抗体与引物交换反应生成正交 DNA 串联体,实现信号高度复用与快速放大,信号放大倍数可达 5 ~ 180 倍。该技术在小鼠视网膜冷冻切片实验中,精准识别 VLP1+calretinin-, VLP1-calretinin+ 和 VLP1+calretinin+ 这 3 种细胞群,在药物-细胞作用机制研究、生物标志物筛选及新药研发等领域具有重要应用价值。 Liu 等 [30] 提出的计算机辅助设计-杂交链式反应( computer-aided design-hybridization chain reaction, CAD-HCR)技术,利用靶标触发等温扩增形成荧光聚集体,经寡核苷酸洗涤链去除后进行下一轮杂交成像。 CAD-HCR产生的荧光信号与靶蛋白丰度呈正相关,且反应条件温和,这种可逆HCR可扩展至活细胞膜蛋白分析,为细胞信号传导、 PPI 网络解析及罕见病诊断提供技术支持。
在蛋白质相互作用研究方面, Raykova 等 [31] 开发的分子布尔逻辑方法,以邻近探针与滚环扩增为信号放大手段,经探针杂交、缺口酶识别、标签侵入、连接、滚环放大等步骤,实现细胞内源性蛋白质相互作用的高灵敏、高特异性可视化检测,有效区分单细胞不同细胞器中的蛋白质。该方法还可通过定量荧光产物数量间接反映蛋白质丰度,为药效评价与精准医疗提供量化依据。 Tao 等 [32] 基于荧光原位杂交( fluorescence in situ hybridization, FISH)原理,开发了高效多重 π-FISH 彩虹方法,通过 4 步 DNA链杂交实现核酸信号逐级放大,并结合荧光探针可视化。将不同单链 DNA 条形码偶联抗体,利用该方法可将蛋白质信号转换为核酸信号,实现靶蛋白超灵敏、高特异性原位多重检测。在 HeLa 细胞检测实验中,该方法成功呈现长链非编码 RNA 核富集丰富转录本 1、 RNA 聚合酶Ⅱ和 MAPK15 基因组位点的空间分布,验证了其多生物分子同时检测能力。
免疫荧光成像技术适用于固定后细胞膜蛋白与胞内蛋白检测,也支持蛋白质与基因组的多模态分析。然而,该技术仍面临多重挑战:受荧光通道限制,难以同时分析大量靶标;多轮次成像耗时较长;图像分析与数据处理复杂度高。未来需进一步优化技术,提升检测速度与通量。
5
基于免疫核酸标签测序的单细胞蛋白质分析技术
近年来,下一代测序( next generation sequencing, NGS)在单细胞领域的应用主要集中于转录组测序 [33]。为全面解析细胞功能与病理状态,将固定细胞或组织的光学成像与 NGS 相结合,可用于蛋白质检测,其检测通量显著高于传统方法。该技术流程为:首先使核酸标签抗体与样品共孵育,完成显微成像后,通过光裂解释放核酸标签并进行测序,进而实现核酸标签与抗体、抗体与抗原的一一对应解码。其中,转录组和表位的细胞标签测序、 RNA 表达和蛋白质测序分析等技术 [34-35],虽然可同时对蛋白质和转录组进行高通量检测,但无法实现蛋白质的亚细胞定位。
为获得空间信息, Merritt 等 [36] 开发了数字空间分析( digital spatial analysis, DSP)平台,支持蛋白质和 RNA 高度多重空间分析。该平台通过光裂解连接子( photocleavable linker, PC linker)将寡核苷酸标签与亲和试剂(抗体或 RNA 探针)偶联,利用特定波长光照射样品特定区域,断裂 linker 键以释放光裂解寡核苷酸。释放的索引寡核苷酸经微毛细管抽吸收集,分配至微量滴度板后,采用 nCounter系统或 NGS 进行数字化读取。该方法检测蛋白质可达单细胞分辨率, RNA 检测限低至约 600 个 mRNA转录本。该研究团队利用 nCounter 系统对淋巴组织、结直肠肿瘤组织和自身免疫组织中 44 种蛋白和 96个基因( 928 个 RNA 探针)进行空间分析,并通过NGS 对 1 412 个基因( 4 998 个 RNA 探针)分析验证了该技术的有效性。进一步应用于黑色素瘤患者临床试验样本,成功鉴定与免疫治疗反应相关的生物标志物,为阐释联合免疫疗法机制提供重要依据。DSP 平台可在 48 小时内收集 384 个样本区域,从组织制备到数据输出的完整流程耗时 1.5 ~ 2.5 天,相比多轮循环成像更自动化、操作更简便。然而,该平台依赖昂贵设备及基于靶向杂交的 mRNA 条形码,灵敏度较低,至少需 20 ~ 300 个细胞方可进行测序。
Sheng 等 [37] 提 出 一 种 组 合 索 引 方 法, 借 助DNA 条形码抗体和分裂池测序技术量化单细胞蛋白质丰度。研究中使用 29 个 DNA 条形码抗体染色10 例血液恶性肿瘤患者临床样本中的外周血单核细胞( peripheral blood mononuclear cell, PBMC),经蛋白表达数据聚类分析显示,特定患者的蛋白丰度与已知病史高度吻合,表明该方法可精准鉴定致病细胞亚群,在复杂样本(如白血病患者样本)检测中极具应用潜力。 Vistain 等 [38] 开发的邻近 测 序( proximity sequencing, Prox-seq) 单 细 胞分析方法,整合单细胞 RNA 测序(single-cell RNA sequencing, scRNA-seq)与邻近连接技术( proximity ligation assay, PLA),可同时测量细胞外蛋白、蛋白复合物和 mRNA。通过计数 PLA 产物,不仅能推断蛋白质丰度、量化基因表达,还可表征不同细胞类型中未知的蛋白质相互作用。该研究团队设计针对 38 个免疫细胞标记物的 Prox-seq 探针对,可测量多达 741 种蛋白质复合物,并在单个 PBMC 中验证了方法的可行性,将单细胞蛋白复合物检测数量从个位数提升至数百个,大幅提高蛋白质检测通量。
总体而言,基于免疫核酸标签测序的技术操作简便,适用于固定后细胞膜蛋白与胞内蛋白检测,当前研究多聚焦于单细胞多模态分析。但该类技术普遍存在亚细胞空间信息丢失的问题,需结合成像等辅助手段以实现单细胞层面的全面分析。
6
基于拉曼光谱的单细胞蛋白分析技术
表 面 增 强 拉 曼 散 射( surface-enhanced Raman scattering, SERS)基于金、银等纳米结构的表面等离子体共振效应,可显著增强拉曼信号。当外加电场作用于金属纳米颗粒( nano-particle, NP)表面时,激发局部表面等离子体共振,使电磁场在金属表面高度富集,产生表面增强效应。样品与该效应相互作用后,可产生强烈的拉曼散射信号,实现高灵敏度检测。
利用 SERS 进行单细胞蛋白分析最简便的方法是直接检测细胞裂解物的 SERS 光谱 [39],但该方法具有破坏性。为实现活细胞检测, Hossain 等 [40] 尝试将 NP 导入细胞,然而 NP 存在均匀性差、重复性低及细胞毒性等问题,无法用于细胞长期监测。
针对上述局限性, Wen 等 [41] 开发了单细胞等离子体免疫夹心法( single-cell plasmonic immunosandwich assay, scPISA),该技术整合体内免疫亲和提取与 SERS,能够定量检测单个活细胞内的信号蛋白及蛋白复合物,其拉曼强度与蛋白丰度呈正相关,可实时评估单细胞水平蛋白表达动态变化。scPISA 具有超高灵敏度,可在单分子水平检测低拷贝数蛋白,仅需约 60 个细胞即可完成检测,且检测耗时短( 15 分钟内)。研究团队以凋亡通路相关蛋白细胞色素 c 和半胱天冬酶-3 为标志物,利用该方法对放线菌素 D、星形孢菌素、二甲双胍等细胞毒性药物进行体外评估,结果与传统方法一致。
为提升蛋白检测通量, Wen 等 [42] 进一步合成了具有明显区分特征峰的拉曼报告分子,开发出多重单细胞等离子免疫夹心法。该方法可同时检测单个活细胞内多种(最多5种)细胞质和细胞核信号蛋白,能够在 10 ~ 24 小时的长时间尺度下,动态解析单细胞内表皮生长因子受体/磷脂酰肌醇 3-激酶信号通路调控机制及其对细胞迁移的影响,为揭示不同迁移表型的分子机制提供了技术支持。
Zhang 等 [43] 提出了一种使用微芯片的电穿孔辅助表面增强拉曼散射方法,将细胞定位、电穿孔和SERS检测集成于同一微芯片,实现对单个活细胞内、外分子的无标记、无创和连续检测。通过对阿霉素处理的耐药及非耐药 MCF-7 和 MDA-MB-231 细胞进行 24 小时连续动态监测,该技术成功阐明了 2 种不同耐药乳腺癌细胞系药物治疗的分子机制。
基于 SERS 的单细胞蛋白分析技术突破了传统裂解或固定细胞检测的限制,可实时动态监测活细胞内蛋白表达变化,支持多次重复取样且不损伤细胞,适用于细胞各部位蛋白质检测,检测速度快(分钟级),有效弥补了其他方法难以分析活细胞靶标动态变化的缺陷。然而,该技术仍存在局限性,如无法获取蛋白质空间分布信息,检测通量有待进一步提升。
上述各技术及相关应用详见表 1。
7
总结与展望
基于免疫的检测方法高度依赖抗体的特异性与亲和力,但市售抗体质量参差不齐,导致检测过程中脱靶效应难以避免;同时,抗体易与其他类似蛋白质发生交叉反应,造成非特异性结合,降低信噪比,过高的背景信号会掩盖靶蛋白信号,因此亟需开发高亲和力且无交叉反应的抗体。此外,实际样本中,许多低丰度蛋白(如信号通路相关磷酸化蛋白)虽在细胞生命活动中发挥关键作用,但其检测面临灵敏度不足的难题。在复杂生物样品中,多种蛋白质协同作用,单一信号蛋白的变化会间接影响其他信号蛋白表达,通过分析多种蛋白表达变化可获取更多信息,因此开发高通量蛋白质检测方法成为当前研究的重要方向。
目前,单细胞蛋白检测技术已应用于蛋白质相互作用表征、肿瘤异质性与耐药机制研究、癌症治疗监测、高通量药物筛选等多个药理学研究领域,但大多仍处于基础研究阶段。主要原因在于,该技术对专业检测人员依赖性强,且所需设备昂贵,严重制约了实际应用推广。因此,研发高特异性、高灵敏度、高通量且低成本的单细胞蛋白质免疫检测技术,成为科研人员的重要目标。此外,单细胞数据具有高维、复杂的特点,实现其与深度学习模型的有效结合,以达成数据分析自动化,也是亟待攻克的难题。同时,单细胞多组学技术的整合联用,将是未来单细胞分析技术的重要发展方向。
参考文献 :
[1] Pe’er D, Ogawa S, Elhanani O, et al. Tumor heterogeneity[J]. Cancer Cell, 2021, 39(8): 1015-1017.
[2] Qi J, Sun H, Zhang Y, et al. Single-cell and spatial analysis reveal interaction of FAP+ fibroblasts and SPP1+ macrophages in colorectal cancer[J]. Nat Commun, 2022, 13(1): 1742.
[3] Shen X, Zhao Y, Wang Z, et al. Recent advances in high-throughput single-cell transcriptomics and spatial transcriptomics[J]. Lab Chip,2022, 22(24): 4774-4791.
[4] Gao S, Shi Q, Zhang Y, et al. Identification of HSC/MPP expansion units in fetal liver by single-cell spatiotemporal transcriptomics[J].Cell Res, 2022, 32(1): 38-53.
[5] Haase C, Gustafsson K, Mei S, et al. Image-seq: spatially resolved single-cell sequencing guided by in situ and in vivo imaging[J]. Nat Methods, 2022, 19(12): 1622-1633.
[6] Russell A J C, Weir J A, Nadaf N M, et al. Slide-tags: scalable, singlenucleus barcoding for multi-modal spatial genomics[PP/OL]. bioRxiv (2023-04-01)[2024-04-16]. https://doi.org/10.1101/2023.04.01.535228.
[7] Stickels R R, Murray E, Kumar P, et al. Highly sensitive spatial transcriptomics at near-cellular resolution with Slide-seqV2[J]. Nat Biotechnol, 2021, 39(3): 313-319.
[8] Zhuang X. Spatially resolved single-cell genomics and transcriptomics by imaging[J]. Nat Methods, 2021, 18(1): 18-22.
[9] Xie H, Ding X. The intriguing landscape of single-cell protein analysis[J]. Adv Sci (Weinh), 2022, 9(12): e2105932.
[10] Liu Y, Herr A E. DropBlot: single-cell western blotting of chemically fixed cancer cells[J]. Nat Commun, 2024, 15(1): 5888.
[11] Long M A, Benman W K J, Petrikas N, et al. Enhancing singlecell Western blotting sensitivity using diffusive analyte blotting and antibody conjugate amplification[J]. Anal Chem, 2023, 95(48):17894-17902.
[12] Lomeli G, Bosse M, Bendall S C, et al. Multiplexed ion beam imaging readout of single-cell immunoblotting[J]. Anal Chem, 2021,93(24): 8517-8525.
[13] Xu L, Xie H, Wang B, et al. Multiplex protein profiling by lowsignal-loss single-cell western blotting with fluorescent-quenching aptamers[J]. Anal Chem, 2023, 95(30): 11399-11409.
[14]Lu Y, Young J, Meng Y G. Electrochemiluminescence to detect surface proteins on live cells[J]. Curr Opin Pharmacol, 2007, 7(5):541-546.
[15] Armbrecht L, Müller R S, Nikoloff J, et al. Single-cell protein profiling in microchambers with barcoded beads[J]. Microsyst Nanoeng, 2019, 5: 55.
[16] Armbrecht L, Rutschmann O, Szczerba B M, et al. Quantification of protein secretion from circulating tumor cells in microfluidic chambers[J]. Adv Sci (Weinh), 2020, 7(11): 1903237.
[17] Yu H, Tai Q, Yang C, et al. Counting protein number in a single cell by a picoliter liquid operating technology[J]. Anal Chem, 2022,94(34): 11925-11933.
[18] Lv S, Zheng D, Chen Z, et al. Near-infrared light-responsive sizeselective lateral flow chip for single-cell manipulation of circulating tumor cells[J]. Anal Chem, 2023, 95(2): 1201-1209.
[19] Bendall S C, Simonds E F, Qiu P, et al. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum[J]. Science, 2011, 332(6030): 687-696.
[20] Giesen C, Wang H A O, Schapiro D, et al. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry[J]. Nat Methods, 2014, 11(4): 417-422.
[21] Baughn L B, Jessen E, Sharma N, et al. Mass Cytometry reveals unique phenotypic patterns associated with subclonal diversity and outcomes in multiple myeloma[J]. Blood Cancer J, 2023, 13(1): 84.
[22] Sheng J, Zhang J, Wang L, et al. Topological analysis of hepatocellular carcinoma tumour microenvironment based on imaging mass cytometry reveals cellular neighbourhood regulated reversely by macrophages with different ontogeny[J]. Gut, 2022, 71(6): 1176-1191.
[23] Zhang Y, Liu P, Majonis D, et al. Polymeric dipicolylamine based mass tags for mass cytometry[J]. Chem Sci, 2022, 13(11): 3233-3243.
[24] Dang J, Li H, Zhang L, et al. New structure mass tag based on ZrNMOF for multiparameter and sensitive single-cell interrogating in mass cytometry[J]. Adv Mater, 2021, 33(35): e2008297.
[25] Chen Y, Wang G, Wang P, et al. Metal-chelatable porphyrinic frameworks for single-cell multiplexing with mass cytometry[J].Angew Chem Int Ed, 2022, 61(38): e202208640.
[26] Liu Z, Yang Y, Zhao X, et al. A universal mass tag based on polystyrene nanoparticles for single-cell multiplexing with mass cytometry[J]. J Colloid Interface Sci, 2023, 639: 434-443.
[27] Wang H, Luo F, Shao X, et al. Integrated proteomics and single-cell mass cytometry analysis dissects the immune landscape of ankylosing spondylitis[J]. Anal Chem, 2023, 95(19): 7702-7714.
[28] Cai S, Hu T, Venkatesan M, et al. Multiplexed protein profiling reveals spatial subcellular signaling networks[J]. iScience, 2022,25(9): 104980.
[29] Saka S K, Wang Y, Kishi J Y, et al. Immuno-SABER enables highly multiplexed and amplified protein imaging in tissues[J]. Nat Biotechnol, 2019, 37(9): 1080-1090.
[30] Liu X, Mao D, Song Y, et al. Computer-aided design of reversible hybridization chain reaction (CAD-HCR) enables multiplexed singlecell spatial proteomics imaging[J]. Sci Adv, 2022, 8(2): eabk0133.
[31] Raykova D, Kermpatsou D, Malmqvist T, et al. A method for Boolean analysis of protein interactions at a molecular level[J]. Nat Commun,2022, 13(1): 4755.
[32] Tao Y, Zhou X, Sun L, et al. Highly efficient and robust π-FISH rainbow for multiplexed in situ detection of diverse biomolecules[J].Nat Commun, 2023, 14(1): 443.
[33] 赵熙萌, 张燕香, 马同辉, 等. 下一代测序技术在癌症诊疗中的应用及研究进展 [J]. 药学进展, 2023, 47(6): 404-416.
[34] Stoeckius M, Hafemeister C, Stephenson W, et al. Simultaneous epitope and transcriptome measurement in single cells[J]. Nat Methods, 2017, 14(9): 865-868.
[35] Peterson V M, Zhang K X, Kumar N, et al. Multiplexed quantification of proteins and transcripts in single cells[J]. Nat Biotechnol, 2017,35(10): 936-939.
[36] Merritt C R, Ong G T, Church S E, et al. Multiplex digital spatial profiling of proteins and RNA in fixed tissue[J]. Nat Biotechnol, 2020,38(5): 586-599.
[37] Sheng J, Hod E A, Vlad G, et al. Quantifying protein abundance on single cells using split-pool sequencing on DNA-barcoded antibodies for diagnostic applications[J]. Sci Rep, 2022, 12(1): 884.
[38] Vistain L, Van Phan H, Keisham B, et al. Quantification of extracellular proteins, protein complexes and mRNAs in single cells by proximity sequencing[J]. Nat Methods, 2022, 19(12): 1578-1589.
[39] Liu S, Su H S, Yang Z, et al. Ag nanorods for label-free surfaceenhanced Raman scattering analysis of cancer cells from cell lysates[J]. ACS Appl Nano Mater, 2021, 5(1): 269-276.
[40] Hossain M K, Cho H Y, Kim K J, et al. In situ monitoring of doxorubicin release from biohybrid nanoparticles modified with antibody and cell-penetrating peptides in breast cancer cells using surface-enhanced raman spectroscopy[J]. Biosens Bioelectron, 2015,71: 300-305.
[41] Wen Y, Liu J, He H, et al. Single-cell analysis of signaling proteins provides insights into proapoptotic properties of anticancer drugs[J].Anal Chem, 2020, 92(18): 12498-12508.
[42] Wen Y, Zhao J, He H, et al. Multiplexed single-cell plasmonic immunoassay of intracellular signaling proteins enables nondestructive monitoring of cell fate[J]. Anal Chem, 2021, 93(42):14204-14213.
[43] Zhang S, Chen S, Zhu R. Electroporation-assisted surface-enhanced raman detection for long-term, label-free, and noninvasive molecular profiling of live single cells[J]. ACS Sens, 2023, 8(2): 555-564.
喜欢我们文章的朋友点个“在看”和“赞”吧,不然微信推送规则改变,有可能每天都会错过我们哦~
免责声明
“汇聚南药”公众号所转载文章来源于其他公众号平台,主要目的在于分享行业相关知识,传递当前最新资讯。图片、文章版权均属于原作者所有,如有侵权,请在留言栏及时告知,我们会在24小时内删除相关信息。
信息来源:药学进展
往期推荐
本平台不对转载文章的观点负责,文章所包含内容的准确性、可靠性或完整性提供任何明示暗示的保证。
免疫疗法诊断试剂
2025-11-21
·药学进展
“
点击蓝字 关注我们
邹秉杰
中国药科大学特聘研究员、博士生导师,药学院药物分析系主任。江苏省杰出青年基金、江苏省“333 人才”、江苏省“六大人才高峰”人才获得者。在 Angew Chem Int Ed, Nucleic Acids Res, Chem Sci, Genome Bio, Biosens Bioelectron 等杂志发表论文 50 余篇。获教育部科技进步一等奖,江苏省医学新技术引进一等奖、二等奖等奖项。担任中国药理学会分析药理学专业委员会常务委员。主要研究方向: 1)核酸与蛋白等生物大分子分析新技术; 2)疾病分子诊断试剂盒研发; 3)单细胞分析新方法。
宋沁馨
中国药科大学药学院药物分析系教授、博士生导师。江苏省 333 工程第三层次培养对象,江苏省青蓝工程中青年学术带头人,中国药理学会分析药理学专委会委员,江苏省药学会药物基因组委员会委员。新西兰皇家科学院、奥克兰大学、维多利亚大学、日本日立中央研究所访问学者,科技部中新科学家交流计划成员。主持国家自然科学基金、江苏省自然科学基金和江苏省社会发展重点项目等纵向课题 20 余项。主编英文、中文专著各 1 部,副主编中文专著 4 部,参编著作 9 部。获教育部科技进步一等奖、江苏省科技进步三等奖、云南省科技进步一等奖、江苏省教育科研成果二等奖、江苏省高校教师教学创新大赛二等奖、南京市优秀论文奖、教育部课程思政教学名师、全国药学专业学位研究生优秀学位论文指导老师、江苏省普通高校优秀毕业论文奖指导老师等奖项和称号。
基于免疫分析法的单细胞蛋白质检测技术研究进展 PPS
邓朝霞,王梦灵,尹王文康,姚春露,张惟杰,王琛,宋沁馨 *,邹秉杰 **
(中国药科大学药学院 药物质量与安全预警教育部重点实验室,江苏 南京 210009)
[ 摘要 ] 单细胞分辨率下解析蛋白质表达,对探究细胞异质性、肿瘤微环境及推进临床精准医学等基础生物学研究具有重要意义。伴随材料化学、核酸化学、蛋白质化学的进步,以及新型检测设备与数据处理系统的迭代升级,单细胞蛋白质检测方法快速发展。其中,基于免疫分析法的单细胞蛋白质检测技术,凭借良好的靶向性和广泛的适用性,成为单细胞蛋白质分析的重要手段。综述了基于免疫分析法的单细胞蛋白质检测技术的原理、优缺点及应用,并对单细胞蛋白质分析技术的发展趋势进行了展望。
细胞是生物体结构与功能的基本单元,除病毒外,几乎所有生物体均由细胞构成。研究机体细胞的功能及其协同机制,对揭示生命本质具有重要意义。尤其在肿瘤研究中,细胞异质性导致肿瘤发生、发展进程及治疗响应存在显著差异 [1]。因此,深入了解肿瘤细胞异质性以及肿瘤微环境中各类细胞的相互作用,是实现癌症精准诊疗的关键。在此背景下,单细胞分析技术应运而生。通过解析单个细胞内功能性分子,该技术能够揭示细胞功能及肿瘤细胞异质性相关机制,为阐明疾病病因、筛选治疗靶点及探索新型治疗模式提供重要依据 [2]。
随着高通量测序技术的进步,多种单细胞基因分析技术相继涌现,包括CEL-seq, MARS-seq, Paired-seq, SMART-seq 和 ScNaUmi-seq 等 [3], 以及用于单细胞空间转录组学分析的 10×Visium[4], Image-seq[5], Slide-tags[6], Slide-seqV2[7], MERFISH和 seqFISH[8] 等。这些技术极大地推动了细胞功能研究,各器官与组织的细胞基因和转录图谱正逐步完善。然而,仅从中心法则上游分子层面解析细胞功能存在局限性,对单细胞内蛋白质等功能大分子的分析,才能更直观地反映细胞的生理状态与功能特性 [9]。因此,单细胞蛋白质分析技术已成为当前研究的重要方向。
目前,单细胞蛋白质分析技术中,除单细胞质谱等少数直接检测方法外,多数依赖基于抗体识别的免疫分析技术。抗体凭借高亲和力和强特异性,能够靶向单细胞内低丰度蛋白质,与传统方法相比,显著降低了检测限,提升了检测灵敏度。通过与流式细胞术、微流控芯片、质谱、纤维荧光成像、拉曼光谱分析等技术联用,免疫分析方法还可实现高通量检测,并获取蛋白质迁移信息,为下游特定表型细胞的分离与分析提供支持。本文将系统综述近年来基于免疫分析法的单细胞蛋白质检测技术的原理、优缺点及其在药理学研究中的应用,并对该领域未来发展趋势进行展望。
1
单细胞蛋白质免疫印迹技术
基于群体细胞的蛋白质印迹法起源于 20 世纪80 年代,现已发展至单细胞水平。该技术通常利用微流控技术分离单个细胞,使其沉降于凝胶中,再经细胞裂解、电泳、转膜、抗体结合及显影等常规蛋白质印迹流程,实现单细胞蛋白质检测。
Liu 等 [10] 开发了液滴印迹法( DropBlot analysis, DropBlot)。该技术通过油包水体系将单个经多聚甲醛固定的细胞封装于微液滴中,利用电转移技术将液滴内细胞裂解物定量转移至聚丙烯酰胺( polyacrylamide, PA)凝胶芯片的微孔内,实现凝胶内单细胞蛋白质免疫印迹( single-cell Western blotting, scWB)。 DropBlot 技术成功检测到保存 6年以上的人源性乳腺癌肿瘤标本中的4种蛋白靶标,且支持在100 ℃高温下孵育1~2小时进行细胞裂解,适用于生物储存库中不同固定条件的细胞样本分析。
然而, scWB 技术仍存在灵敏度不足和检测通量低的问题。为降低检测限, Long 等 [11] 将电泳分离与抗体检测过程解耦,先将蛋白质转移至硝酸纤维素膜,再使用酶标抗体进行检测,有效放大检测信号,实现低丰度靶标的检测。应用该改进方法检测表达增强型绿色荧光蛋白( enhanced green fluorescent protein, EGFP)的 HEK293 T 细胞,阳性检出率达 100%,较传统凝胶内检测方法提高了15.5 个百分点。针对 scWB 重复抗体剥离过程限制多重蛋白检测的问题, Lomeli 等 [12] 开发了基于飞行时间多路离子束成像的单细胞免疫印迹技术( multiplexed ion beam imaging by time-of-flight of single-cell immunoblotting, scIB-MIBI-TOF)。该技术采用金属标记抗体,避免光谱重叠,将单次可检测蛋白种类从约 3 种提升至约 40 种,大幅提高检测通量,并成功检测到 U251-tGFP 细胞中 2 种不同形式的 EGFP。该技术拓展应用于免疫细胞和耐药相关蛋白检测,有望用于预测异质肿瘤的耐药性。 Xu等 [13] 则提出基于荧光-淬灭适配体的单细胞蛋白质印 迹 法( fluorescent-quenching aptamer-based singlecell Western blotting, FAS-WB),设计了一对分别标记荧光基团和淬灭基团的互补适配体。每轮成像由荧光适配体识别靶标,成像后通过杂交互补适配体淬灭荧光信号,实现连续检测。该迭代淬灭策略突破了多重检测限制,省去抗体剥离步骤,避免信号损失;同时,适配体因尺寸小,可更快渗透进入PA 凝胶。 FAS-WB 每个检测周期不到 25 分钟,成本仅为传统抗体探针的 1/1 000,显著提升了检测效率。通过同时检测 Lncap 细胞内癌抗原 125、上皮细胞黏附分子、蛋白酪氨酸激酶 7、人表皮生长因子受体 2( human epidermal growth factor receptor 2, HER2)这 4 种蛋白,验证了该方法的有效性。
尽管上述改进在一定程度上提升了单细胞蛋白质检测的灵敏度和通量,但 scWB 技术仅适用于裂解后的细胞,无法保留细胞的完整性及位置信息,限制了其在临床领域的应用。
2
基于微流控的单细胞免疫检测技术
细胞内多数蛋白质丰度较低,亟需高灵敏度和高特异性的分析方法。近年来,微流控设备与电化学发光、荧光免疫(微珠或固相载体)、侧流免疫层析等免疫检测平台的融合,使微型免疫检测技术成为研究热点。如典型的荧光微流控免疫分析法通过微流控设备将单个细胞捕获至独立微室,利用微室中的荧光条形码免疫磁珠,实现细胞表面蛋白或裂解后胞内蛋白的检测,根据荧光颜色和强度即可解码出蛋白种类与丰度。
Lu 等 [14] 基于钌标记抗体在三丙胺存在下于电极表面氧化发光的原理,开发了电化学发光检测系统,用于活细胞表面蛋白检测。研究发现,经肿瘤坏死因子 α、淋巴素( lymphonectin, LT) α3 和LTα1β2 等活化剂刺激后,人脐静脉内皮细胞的细胞间黏附分子-1 表达显著上调,与预期相符。该方法背景信号低,信噪比高,无需繁琐的细胞分离步骤,避免了分离过程中细胞表面蛋白的改变,但检测蛋白通量仍存在局限。
相对于电化学法,荧光免疫法因检测通量高而备受青睐。 Armbrecht 等 [15] 构建了含 1 026 个微室(单室体积 152 pL)的微流控平台,可同时捕获单细胞与荧光条形码免疫磁珠。细胞裂解后,释放的蛋白与磁珠结合,通过磁珠荧光特征实现蛋白定性定量分析。该团队应用此平台对 MCF-7, SK-BR-3和 HEK-293T 细胞内甘油醛-3- 磷酸脱氢酶、半乳糖凝集素-3 和半乳糖凝集素-3 结合蛋白进行多重免疫测定,结果与高通量 RNA 测序数据一致,检测限低至 1.8×103 个分子。后续研究通过增设物理控制阀 [16] 优化芯片结构,实现全血中循环肿瘤细胞( circulating tumor cell, CTC)的物理分离,在检测乳腺癌单个 CTC 分泌的粒细胞生长刺激因子时,检测限达 1.5 μg·L-1,灵敏度和特异性优异,细胞捕获效率达95%以上,集成化设计显著提升临床样本检测效率。
为实现单细胞蛋白的高灵敏检测, Yu 等 [17] 结合普通荧光显微镜、自制毛细管皮升操作设备及气泡黏附细胞取样技术,开发了单细胞皮升液体操 作 技 术( single-cell picoliter liquid operating technology, scPILOT)。该技术采用量子点标记抗体微阵列,通过显微成像计数单细胞中超低拷贝数蛋白,可在 50 pL 反应单元内检测单细胞中 1 ~ 1 500 个拷贝的超低丰度蛋白,线性与重复性良好。应用该技术检测三阴性乳腺癌细胞中 HER2 蛋白,揭示其表达存在显著细胞间异质性,为信号网络中关键蛋白的验证和定量提供了有力工具。 Lv 等 [18] 设计了近红外光( near-infrared light, NIR)响应微流控芯片,整合尺寸选择性侧流微阵列与金纳米棒预嵌明胶( NIR 响应水凝胶)捕获单元,实现高纯度、高活力单 CTC 的捕获与位点特异性释放。临床检测结果显示,该芯片可从 11 名癌症(乳腺癌、肺癌、结肠癌)患者血液中成功捕获 CTC,而 5 名健康对照样本均呈阴性,验证了其临床应用价值。
总体而言,微流控芯片与免疫分析法结合的单细胞蛋白质检测技术,兼具裂解细胞与完整活细胞检测能力,可覆盖胞内蛋白、膜蛋白及分泌蛋白检测,在临床药理学研究中优势显著。该技术操作简便,可自动化完成单细胞分离与分析,但受荧光通道数量限制,检测靶标通量仍有待提升,且难以提供蛋白质在细胞内的空间分布信息。
3
质谱流式细胞技术
质谱流式细胞术( mass cytometry, MC)是基于质谱原理的单细胞多参数检测技术,通过过渡元素标记抗体,利用抗体和待测样品的免疫结合,最终借助电感耦合等离子体-质谱( inductively coupled plasma-mass spectrometry, ICP-MS)进行检测。该技术最早被称为飞行时间质谱流式细胞术( cytometry by time-of-flight mass spectrometry, CyTOF),目前主要分为悬浮质谱流式细胞术( suspension mass cytometry, SMC)和成像质谱流式细胞术( imaging mass cytometry, IMC)两大类。
Bendall 等 [19] 使用过渡金属同位素标记抗体,将结合抗体-同位素偶联物的细胞以单细胞液滴形式喷入约 5 500 K 的电感耦合氩等离子体中,促使细胞蒸发并产生原子电离,随后利用飞行时间质谱仪对元素离子进行采样和定量分析。该方法可同时测定单个人骨髓单核细胞中的 31 种靶标蛋白,突破了荧光通道限制,为解析健康人造血系统免疫信号提供了全面视角,标志着 SMC 在单细胞生物学研究中的重要突破。为获取空间信息, Giesen 等 [20]将免疫细胞化学与高分辨率激光消融技术联合应用于 CyTOF,开发出 IMC,可在亚细胞分辨率下同时成像 32 种蛋白质及其修饰。利用该技术分析磷酸酪氨酸磷酸酶抑制剂处理的 HMLE 细胞,发现丝裂原活化蛋白激酶( mitogen-activated protein kinase, MAPK)通路和 Janus 激酶-信号转导与转录激活因 子( Janus kinase-signal transducer and activator of transcription, JAK-STAT)通路相关蛋白,如细胞外信号调节激酶(extracellular signal-regulated kinase, ERK) 1 的 T202 和 Y204 位 点、 ERK2 的 T184 和Y186 位点、 p38 丝裂原活化蛋白激酶(p38 mitogenactivated protein kinase, p38MAPK)的 T180 和 Y182位点、 STAT5 的 Y694 位点磷酸化水平显著升高,而核因子-κB 通路、 AMP 活化蛋白激酶通路等非酪氨酸磷酸化位点无明显变化。此外, IMC 应用于人类乳腺癌样本时,能够表征细胞亚群、细胞间相互作用及肿瘤异质性。 Baughn 等 [21] 采用 SMC 方法,对 49 份新诊断或复发/难治性多发性骨髓瘤( multiple myeloma, MM)患者的原发性肿瘤样本进行分析,检测 34 种靶标,鉴定出 13 个表型元簇,绘制出原发性MM首份大规模单细胞蛋白质图谱。 Sheng等 [22]设计高复用 IMC 检测面板,量化 134 名肝癌患者及7 名健康供体样本中的 36 种组织生物标志物,生成562 张单细胞分辨率的高复用组织学图像,首次揭示肝癌肿瘤微环境的多种拓扑功能单元。
尽管 MC 技术显著提升了靶标检测通量并实现了对蛋白位置信息的获取,但其仍存在局限性:其一,检测能力受金属同位素数目的限制,难以达到组学水平的多重检测;其二,金属同位素标记抗体成本高昂,导致检测费用居高不下。
为了克服以上问题,研究者开发了多种质量标签试剂,包括金属螯合聚合物( metal chelating polymer, MCP)、小分子质量标签、聚合物微珠及纳米粒子标签等。 Zhang 等 [23] 通过巯基-马来酰亚胺反应和叠氮-二苯并环辛炔点击反应,在铂/二苯胺复合物的悬垂基团上引入低聚乙二醇(polyethylene glycol, PEG)合成制得新型铼质量标签,解决了传统 MCP 标签仅能结合硬金属离子及易发生聚合物沉淀的问题;该聚合物标签可与商用金属偶联抗体协同用于多参数单细胞免疫检测,拓展了质量标签的应用范围。 Dang 等 [24] 报道了基于纳米金属有机框架( nanometal organic framework, NMOF)的Zr-NMOF 质量标签,直径约 33 nm,在水中分散均匀且稳定性高(储存期超 1 年),信号放大倍数较MCP 标签增加 5 倍,适用于 CyTOF 中的高灵敏和高通量单细胞生物标志物检测,且与其他质量标签兼容性良好。 Chen 等 [25] 开发基于 PEG 功能化的介孔 卟 啉 框 架(mesoporous porphyrinic framework, MPF)用于 SMC 分析,该 MPF 可螯合 Sn, W, Pt, Pd 等 27 种非镧系金属稳定同位素,有效扩展了 CyTOF 的检测通道,克服了传统 MCP 材料中负载金属种类有限的缺点。 Liu 等 [26] 采用聚苯乙烯纳米颗粒这一极易得的经典材料作为金属载体,通过优化细胞染色缓冲液降低非特异性结合,灵敏度较传统 MCP 标签提高 5 倍,为增加检测通道提供了低成本方案。
随着 MC 技术的发展,其与其他技术的联用在临床研究中展现出重要价值。 Wang 等 [27] 将 SMC与基于质谱的无标记单细胞蛋白质组学进行整合,探 究 了 强 直 性 脊 柱 炎( ankylosing spondylitis, AS)的免疫特征。该研究纳入 44 名未经治疗的AS 患者、 14 名经非甾体抗炎药和抗风湿药物治疗的 AS 患者和 31 名健康个体,揭示出 32 种免疫标记物与细胞表型的临床关联,为系统地了解 AS 的发病机制提供了新的见解,有助于进一步开发新的治疗策略。
目前, MC 技术可检测裂解细胞及固定细胞,适用于细胞内各类蛋白质检测,已在医院、高校等科研机构逐步应用。然而,昂贵的仪器设备和较高的分析成本,限制了其更广泛的普及与应用。
4
免疫荧光显微成像技术
免疫荧光常与荧光显微镜联用,以成像方式呈现数据。其优势在于能够以亚细胞分辨率提供细胞位置信息,结合邻近技术,可间接推断蛋白-蛋白相互作用( protein-protein interaction, PPI),直观反映细胞生理过程。免疫荧光主要有 3 种模式:直接使用荧光标记一抗检测靶蛋白;通过荧光标记二抗间接放大一抗信号;新兴的荧光标记核酸法则为抗体添加核酸标签,利用核酸的可编程性和可扩增性,将蛋白质信号转化为核酸信号,显著提升检测灵敏度和检测通量。
Cai 等 [28] 开发的快速多重免疫荧光法,利用特殊的可重复标记染料,突破传统荧光显微镜通道限制,实现 25 种标记物同时成像。通过单像素分析,该方法成功揭示了 WNT/β-连环蛋白通路和 v-akt 鼠胸腺瘤病毒癌基因同源物编码蛋白/哺乳动物雷帕霉素靶蛋白通路中信号蛋白的表达水平与核-质易位现象,解析了激酶的磷酸化活性及其在信号通路中的调控作用。针对染料直标抗体检测低丰度蛋白灵敏度不足的问题, Saka 等 [29] 提出通过交换反应信号放大的免疫染色( immunostaining with signal amplification by exchange reaction, Immuno-SABER)技术,通过 DNA 条形码抗体与引物交换反应生成正交 DNA 串联体,实现信号高度复用与快速放大,信号放大倍数可达 5 ~ 180 倍。该技术在小鼠视网膜冷冻切片实验中,精准识别 VLP1+calretinin-, VLP1-calretinin+ 和 VLP1+calretinin+ 这 3 种细胞群,在药物-细胞作用机制研究、生物标志物筛选及新药研发等领域具有重要应用价值。 Liu 等 [30] 提出的计算机辅助设计-杂交链式反应( computer-aided design-hybridization chain reaction, CAD-HCR)技术,利用靶标触发等温扩增形成荧光聚集体,经寡核苷酸洗涤链去除后进行下一轮杂交成像。 CAD-HCR产生的荧光信号与靶蛋白丰度呈正相关,且反应条件温和,这种可逆HCR可扩展至活细胞膜蛋白分析,为细胞信号传导、 PPI 网络解析及罕见病诊断提供技术支持。
在蛋白质相互作用研究方面, Raykova 等 [31] 开发的分子布尔逻辑方法,以邻近探针与滚环扩增为信号放大手段,经探针杂交、缺口酶识别、标签侵入、连接、滚环放大等步骤,实现细胞内源性蛋白质相互作用的高灵敏、高特异性可视化检测,有效区分单细胞不同细胞器中的蛋白质。该方法还可通过定量荧光产物数量间接反映蛋白质丰度,为药效评价与精准医疗提供量化依据。 Tao 等 [32] 基于荧光原位杂交( fluorescence in situ hybridization, FISH)原理,开发了高效多重 π-FISH 彩虹方法,通过 4 步 DNA链杂交实现核酸信号逐级放大,并结合荧光探针可视化。将不同单链 DNA 条形码偶联抗体,利用该方法可将蛋白质信号转换为核酸信号,实现靶蛋白超灵敏、高特异性原位多重检测。在 HeLa 细胞检测实验中,该方法成功呈现长链非编码 RNA 核富集丰富转录本 1、 RNA 聚合酶Ⅱ和 MAPK15 基因组位点的空间分布,验证了其多生物分子同时检测能力。
免疫荧光成像技术适用于固定后细胞膜蛋白与胞内蛋白检测,也支持蛋白质与基因组的多模态分析。然而,该技术仍面临多重挑战:受荧光通道限制,难以同时分析大量靶标;多轮次成像耗时较长;图像分析与数据处理复杂度高。未来需进一步优化技术,提升检测速度与通量。
5
基于免疫核酸标签测序的单细胞蛋白质分析技术
近年来,下一代测序( next generation sequencing, NGS)在单细胞领域的应用主要集中于转录组测序 [33]。为全面解析细胞功能与病理状态,将固定细胞或组织的光学成像与 NGS 相结合,可用于蛋白质检测,其检测通量显著高于传统方法。该技术流程为:首先使核酸标签抗体与样品共孵育,完成显微成像后,通过光裂解释放核酸标签并进行测序,进而实现核酸标签与抗体、抗体与抗原的一一对应解码。其中,转录组和表位的细胞标签测序、 RNA 表达和蛋白质测序分析等技术 [34-35],虽然可同时对蛋白质和转录组进行高通量检测,但无法实现蛋白质的亚细胞定位。
为获得空间信息, Merritt 等 [36] 开发了数字空间分析( digital spatial analysis, DSP)平台,支持蛋白质和 RNA 高度多重空间分析。该平台通过光裂解连接子( photocleavable linker, PC linker)将寡核苷酸标签与亲和试剂(抗体或 RNA 探针)偶联,利用特定波长光照射样品特定区域,断裂 linker 键以释放光裂解寡核苷酸。释放的索引寡核苷酸经微毛细管抽吸收集,分配至微量滴度板后,采用 nCounter系统或 NGS 进行数字化读取。该方法检测蛋白质可达单细胞分辨率, RNA 检测限低至约 600 个 mRNA转录本。该研究团队利用 nCounter 系统对淋巴组织、结直肠肿瘤组织和自身免疫组织中 44 种蛋白和 96个基因( 928 个 RNA 探针)进行空间分析,并通过NGS 对 1 412 个基因( 4 998 个 RNA 探针)分析验证了该技术的有效性。进一步应用于黑色素瘤患者临床试验样本,成功鉴定与免疫治疗反应相关的生物标志物,为阐释联合免疫疗法机制提供重要依据。DSP 平台可在 48 小时内收集 384 个样本区域,从组织制备到数据输出的完整流程耗时 1.5 ~ 2.5 天,相比多轮循环成像更自动化、操作更简便。然而,该平台依赖昂贵设备及基于靶向杂交的 mRNA 条形码,灵敏度较低,至少需 20 ~ 300 个细胞方可进行测序。
Sheng 等 [37] 提 出 一 种 组 合 索 引 方 法, 借 助DNA 条形码抗体和分裂池测序技术量化单细胞蛋白质丰度。研究中使用 29 个 DNA 条形码抗体染色10 例血液恶性肿瘤患者临床样本中的外周血单核细胞( peripheral blood mononuclear cell, PBMC),经蛋白表达数据聚类分析显示,特定患者的蛋白丰度与已知病史高度吻合,表明该方法可精准鉴定致病细胞亚群,在复杂样本(如白血病患者样本)检测中极具应用潜力。 Vistain 等 [38] 开发的邻近 测 序( proximity sequencing, Prox-seq) 单 细 胞分析方法,整合单细胞 RNA 测序(single-cell RNA sequencing, scRNA-seq)与邻近连接技术( proximity ligation assay, PLA),可同时测量细胞外蛋白、蛋白复合物和 mRNA。通过计数 PLA 产物,不仅能推断蛋白质丰度、量化基因表达,还可表征不同细胞类型中未知的蛋白质相互作用。该研究团队设计针对 38 个免疫细胞标记物的 Prox-seq 探针对,可测量多达 741 种蛋白质复合物,并在单个 PBMC 中验证了方法的可行性,将单细胞蛋白复合物检测数量从个位数提升至数百个,大幅提高蛋白质检测通量。
总体而言,基于免疫核酸标签测序的技术操作简便,适用于固定后细胞膜蛋白与胞内蛋白检测,当前研究多聚焦于单细胞多模态分析。但该类技术普遍存在亚细胞空间信息丢失的问题,需结合成像等辅助手段以实现单细胞层面的全面分析。
6
基于拉曼光谱的单细胞蛋白分析技术
表 面 增 强 拉 曼 散 射( surface-enhanced Raman scattering, SERS)基于金、银等纳米结构的表面等离子体共振效应,可显著增强拉曼信号。当外加电场作用于金属纳米颗粒( nano-particle, NP)表面时,激发局部表面等离子体共振,使电磁场在金属表面高度富集,产生表面增强效应。样品与该效应相互作用后,可产生强烈的拉曼散射信号,实现高灵敏度检测。
利用 SERS 进行单细胞蛋白分析最简便的方法是直接检测细胞裂解物的 SERS 光谱 [39],但该方法具有破坏性。为实现活细胞检测, Hossain 等 [40] 尝试将 NP 导入细胞,然而 NP 存在均匀性差、重复性低及细胞毒性等问题,无法用于细胞长期监测。
针对上述局限性, Wen 等 [41] 开发了单细胞等离子体免疫夹心法( single-cell plasmonic immunosandwich assay, scPISA),该技术整合体内免疫亲和提取与 SERS,能够定量检测单个活细胞内的信号蛋白及蛋白复合物,其拉曼强度与蛋白丰度呈正相关,可实时评估单细胞水平蛋白表达动态变化。scPISA 具有超高灵敏度,可在单分子水平检测低拷贝数蛋白,仅需约 60 个细胞即可完成检测,且检测耗时短( 15 分钟内)。研究团队以凋亡通路相关蛋白细胞色素 c 和半胱天冬酶-3 为标志物,利用该方法对放线菌素 D、星形孢菌素、二甲双胍等细胞毒性药物进行体外评估,结果与传统方法一致。
为提升蛋白检测通量, Wen 等 [42] 进一步合成了具有明显区分特征峰的拉曼报告分子,开发出多重单细胞等离子免疫夹心法。该方法可同时检测单个活细胞内多种(最多5种)细胞质和细胞核信号蛋白,能够在 10 ~ 24 小时的长时间尺度下,动态解析单细胞内表皮生长因子受体/磷脂酰肌醇 3-激酶信号通路调控机制及其对细胞迁移的影响,为揭示不同迁移表型的分子机制提供了技术支持。
Zhang 等 [43] 提出了一种使用微芯片的电穿孔辅助表面增强拉曼散射方法,将细胞定位、电穿孔和SERS检测集成于同一微芯片,实现对单个活细胞内、外分子的无标记、无创和连续检测。通过对阿霉素处理的耐药及非耐药 MCF-7 和 MDA-MB-231 细胞进行 24 小时连续动态监测,该技术成功阐明了 2 种不同耐药乳腺癌细胞系药物治疗的分子机制。
基于 SERS 的单细胞蛋白分析技术突破了传统裂解或固定细胞检测的限制,可实时动态监测活细胞内蛋白表达变化,支持多次重复取样且不损伤细胞,适用于细胞各部位蛋白质检测,检测速度快(分钟级),有效弥补了其他方法难以分析活细胞靶标动态变化的缺陷。然而,该技术仍存在局限性,如无法获取蛋白质空间分布信息,检测通量有待进一步提升。
上述各技术及相关应用详见表 1。
7
总结与展望
基于免疫的检测方法高度依赖抗体的特异性与亲和力,但市售抗体质量参差不齐,导致检测过程中脱靶效应难以避免;同时,抗体易与其他类似蛋白质发生交叉反应,造成非特异性结合,降低信噪比,过高的背景信号会掩盖靶蛋白信号,因此亟需开发高亲和力且无交叉反应的抗体。此外,实际样本中,许多低丰度蛋白(如信号通路相关磷酸化蛋白)虽在细胞生命活动中发挥关键作用,但其检测面临灵敏度不足的难题。在复杂生物样品中,多种蛋白质协同作用,单一信号蛋白的变化会间接影响其他信号蛋白表达,通过分析多种蛋白表达变化可获取更多信息,因此开发高通量蛋白质检测方法成为当前研究的重要方向。
目前,单细胞蛋白检测技术已应用于蛋白质相互作用表征、肿瘤异质性与耐药机制研究、癌症治疗监测、高通量药物筛选等多个药理学研究领域,但大多仍处于基础研究阶段。主要原因在于,该技术对专业检测人员依赖性强,且所需设备昂贵,严重制约了实际应用推广。因此,研发高特异性、高灵敏度、高通量且低成本的单细胞蛋白质免疫检测技术,成为科研人员的重要目标。此外,单细胞数据具有高维、复杂的特点,实现其与深度学习模型的有效结合,以达成数据分析自动化,也是亟待攻克的难题。同时,单细胞多组学技术的整合联用,将是未来单细胞分析技术的重要发展方向。
参考文献 :
[1] Pe’er D, Ogawa S, Elhanani O, et al. Tumor heterogeneity[J]. Cancer Cell, 2021, 39(8): 1015-1017.
[2] Qi J, Sun H, Zhang Y, et al. Single-cell and spatial analysis reveal interaction of FAP+ fibroblasts and SPP1+ macrophages in colorectal cancer[J]. Nat Commun, 2022, 13(1): 1742.
[3] Shen X, Zhao Y, Wang Z, et al. Recent advances in high-throughput single-cell transcriptomics and spatial transcriptomics[J]. Lab Chip,2022, 22(24): 4774-4791.
[4] Gao S, Shi Q, Zhang Y, et al. Identification of HSC/MPP expansion units in fetal liver by single-cell spatiotemporal transcriptomics[J].Cell Res, 2022, 32(1): 38-53.
[5] Haase C, Gustafsson K, Mei S, et al. Image-seq: spatially resolved single-cell sequencing guided by in situ and in vivo imaging[J]. Nat Methods, 2022, 19(12): 1622-1633.
[6] Russell A J C, Weir J A, Nadaf N M, et al. Slide-tags: scalable, singlenucleus barcoding for multi-modal spatial genomics[PP/OL]. bioRxiv (2023-04-01)[2024-04-16]. https://doi.org/10.1101/2023.04.01.535228.
[7] Stickels R R, Murray E, Kumar P, et al. Highly sensitive spatial transcriptomics at near-cellular resolution with Slide-seqV2[J]. Nat Biotechnol, 2021, 39(3): 313-319.
[8] Zhuang X. Spatially resolved single-cell genomics and transcriptomics by imaging[J]. Nat Methods, 2021, 18(1): 18-22.
[9] Xie H, Ding X. The intriguing landscape of single-cell protein analysis[J]. Adv Sci (Weinh), 2022, 9(12): e2105932.
[10] Liu Y, Herr A E. DropBlot: single-cell western blotting of chemically fixed cancer cells[J]. Nat Commun, 2024, 15(1): 5888.
[11] Long M A, Benman W K J, Petrikas N, et al. Enhancing singlecell Western blotting sensitivity using diffusive analyte blotting and antibody conjugate amplification[J]. Anal Chem, 2023, 95(48):17894-17902.
[12] Lomeli G, Bosse M, Bendall S C, et al. Multiplexed ion beam imaging readout of single-cell immunoblotting[J]. Anal Chem, 2021,93(24): 8517-8525.
[13] Xu L, Xie H, Wang B, et al. Multiplex protein profiling by lowsignal-loss single-cell western blotting with fluorescent-quenching aptamers[J]. Anal Chem, 2023, 95(30): 11399-11409.
[14]Lu Y, Young J, Meng Y G. Electrochemiluminescence to detect surface proteins on live cells[J]. Curr Opin Pharmacol, 2007, 7(5):541-546.
[15] Armbrecht L, Müller R S, Nikoloff J, et al. Single-cell protein profiling in microchambers with barcoded beads[J]. Microsyst Nanoeng, 2019, 5: 55.
[16] Armbrecht L, Rutschmann O, Szczerba B M, et al. Quantification of protein secretion from circulating tumor cells in microfluidic chambers[J]. Adv Sci (Weinh), 2020, 7(11): 1903237.
[17] Yu H, Tai Q, Yang C, et al. Counting protein number in a single cell by a picoliter liquid operating technology[J]. Anal Chem, 2022,94(34): 11925-11933.
[18] Lv S, Zheng D, Chen Z, et al. Near-infrared light-responsive sizeselective lateral flow chip for single-cell manipulation of circulating tumor cells[J]. Anal Chem, 2023, 95(2): 1201-1209.
[19] Bendall S C, Simonds E F, Qiu P, et al. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum[J]. Science, 2011, 332(6030): 687-696.
[20] Giesen C, Wang H A O, Schapiro D, et al. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry[J]. Nat Methods, 2014, 11(4): 417-422.
[21] Baughn L B, Jessen E, Sharma N, et al. Mass Cytometry reveals unique phenotypic patterns associated with subclonal diversity and outcomes in multiple myeloma[J]. Blood Cancer J, 2023, 13(1): 84.
[22] Sheng J, Zhang J, Wang L, et al. Topological analysis of hepatocellular carcinoma tumour microenvironment based on imaging mass cytometry reveals cellular neighbourhood regulated reversely by macrophages with different ontogeny[J]. Gut, 2022, 71(6): 1176-1191.
[23] Zhang Y, Liu P, Majonis D, et al. Polymeric dipicolylamine based mass tags for mass cytometry[J]. Chem Sci, 2022, 13(11): 3233-3243.
[24] Dang J, Li H, Zhang L, et al. New structure mass tag based on ZrNMOF for multiparameter and sensitive single-cell interrogating in mass cytometry[J]. Adv Mater, 2021, 33(35): e2008297.
[25] Chen Y, Wang G, Wang P, et al. Metal-chelatable porphyrinic frameworks for single-cell multiplexing with mass cytometry[J].Angew Chem Int Ed, 2022, 61(38): e202208640.
[26] Liu Z, Yang Y, Zhao X, et al. A universal mass tag based on polystyrene nanoparticles for single-cell multiplexing with mass cytometry[J]. J Colloid Interface Sci, 2023, 639: 434-443.
[27] Wang H, Luo F, Shao X, et al. Integrated proteomics and single-cell mass cytometry analysis dissects the immune landscape of ankylosing spondylitis[J]. Anal Chem, 2023, 95(19): 7702-7714.
[28] Cai S, Hu T, Venkatesan M, et al. Multiplexed protein profiling reveals spatial subcellular signaling networks[J]. iScience, 2022,25(9): 104980.
[29] Saka S K, Wang Y, Kishi J Y, et al. Immuno-SABER enables highly multiplexed and amplified protein imaging in tissues[J]. Nat Biotechnol, 2019, 37(9): 1080-1090.
[30] Liu X, Mao D, Song Y, et al. Computer-aided design of reversible hybridization chain reaction (CAD-HCR) enables multiplexed singlecell spatial proteomics imaging[J]. Sci Adv, 2022, 8(2): eabk0133.
[31] Raykova D, Kermpatsou D, Malmqvist T, et al. A method for Boolean analysis of protein interactions at a molecular level[J]. Nat Commun,2022, 13(1): 4755.
[32] Tao Y, Zhou X, Sun L, et al. Highly efficient and robust π-FISH rainbow for multiplexed in situ detection of diverse biomolecules[J].Nat Commun, 2023, 14(1): 443.
[33] 赵熙萌, 张燕香, 马同辉, 等. 下一代测序技术在癌症诊疗中的应用及研究进展 [J]. 药学进展, 2023, 47(6): 404-416.
[34] Stoeckius M, Hafemeister C, Stephenson W, et al. Simultaneous epitope and transcriptome measurement in single cells[J]. Nat Methods, 2017, 14(9): 865-868.
[35] Peterson V M, Zhang K X, Kumar N, et al. Multiplexed quantification of proteins and transcripts in single cells[J]. Nat Biotechnol, 2017,35(10): 936-939.
[36] Merritt C R, Ong G T, Church S E, et al. Multiplex digital spatial profiling of proteins and RNA in fixed tissue[J]. Nat Biotechnol, 2020,38(5): 586-599.
[37] Sheng J, Hod E A, Vlad G, et al. Quantifying protein abundance on single cells using split-pool sequencing on DNA-barcoded antibodies for diagnostic applications[J]. Sci Rep, 2022, 12(1): 884.
[38] Vistain L, Van Phan H, Keisham B, et al. Quantification of extracellular proteins, protein complexes and mRNAs in single cells by proximity sequencing[J]. Nat Methods, 2022, 19(12): 1578-1589.
[39] Liu S, Su H S, Yang Z, et al. Ag nanorods for label-free surfaceenhanced Raman scattering analysis of cancer cells from cell lysates[J]. ACS Appl Nano Mater, 2021, 5(1): 269-276.
[40] Hossain M K, Cho H Y, Kim K J, et al. In situ monitoring of doxorubicin release from biohybrid nanoparticles modified with antibody and cell-penetrating peptides in breast cancer cells using surface-enhanced raman spectroscopy[J]. Biosens Bioelectron, 2015,71: 300-305.
[41] Wen Y, Liu J, He H, et al. Single-cell analysis of signaling proteins provides insights into proapoptotic properties of anticancer drugs[J].Anal Chem, 2020, 92(18): 12498-12508.
[42] Wen Y, Zhao J, He H, et al. Multiplexed single-cell plasmonic immunoassay of intracellular signaling proteins enables nondestructive monitoring of cell fate[J]. Anal Chem, 2021, 93(42):14204-14213.
[43] Zhang S, Chen S, Zhu R. Electroporation-assisted surface-enhanced raman detection for long-term, label-free, and noninvasive molecular profiling of live single cells[J]. ACS Sens, 2023, 8(2): 555-564.
美编排版:覃冰冰
感谢您阅读《药学进展》微信平台原创好文,也欢迎各位读者转载、引用。本文选自《药学进展》2025年第 8 期。
《药学进展》杂志由教育部主管、中国药科大学主办,中国科技核心期刊(中国科技论文统计源期刊)。刊物以反映药学科研领域的新方法、新成果、新进展、新趋势为宗旨,以综述、评述、行业发展报告为特色,以药学学科进展、技术进展、新药研发各环节技术信息为重点,是一本专注于医药科技前沿与产业动态的专业媒体。
《药学进展》注重内容策划、加强组稿约稿、深度挖掘、分析药学信息资源、在药学学科进展、科研思路方法、靶点机制探讨、新药研发报告、临床用药分析、国际医药前沿等方面初具特色;特别是医药信息内容以科学前沿与国家战略需求相合,更加突出前瞻性、权威性、时效性、新颖性、系统性、实战性。根据最新统计数据,刊物篇均下载率连续三年蝉联我国医药期刊榜首,复合影响因子1.216,具有较高的影响力。
《药学进展》编委会由国家重大专项化学药总师陈凯先院士担任主编,编委由新药研发技术链政府监管部门、高校科研院所、制药企业、临床医院、CRO、金融资本及知识产权相关机构近两百位极具影响力的专家组成。
联系《药学进展》↓↓↓
编辑部官网:pps.cpu.edu.cn;
邮箱:yxjz@163.com;
电话:025-83271227。
欢迎投稿、订阅!
往期推荐
聚焦“第七届药学前沿学术论坛暨《药学进展》编委会会议”
聚力前沿赛道,共启学术新程:第七届药学前沿学术论坛在南京隆重启幕
承学术匠心,启时代新章:《药学进展》第七届编委会、第三届青年编委会成立大会在宁召开
聚焦“兴药为民·2023生物医药创新融合发展大会”“兴药为民·2023生物医药创新融合发展大会”盛大启幕!院士专家齐聚杭城,绘就生物医药前沿赛道新蓝图“兴药强刊”青年学者论坛暨《药学进展》第二届青年编委会议成功召开“兴药为民·2023生物医药创新融合发展大会”路演专场圆满收官!校企合作新旅程已启航
我知道你在看哟
100 项与 Auckland University 相关的药物交易
登录后查看更多信息
100 项与 Auckland University 相关的转化医学
登录后查看更多信息
组织架构
使用我们的机构树数据加速您的研究。
登录
或

管线布局
2026年03月01日管线快照
管线布局中药物为当前组织机构及其子机构作为药物机构进行统计,早期临床1期并入临床1期,临床1/2期并入临床2期,临床2/3期并入临床3期
药物发现
3
12
临床前
其他
15
登录后查看更多信息
当前项目
登录后查看更多信息
药物交易
使用我们的药物交易数据加速您的研究。
登录
或

转化医学
使用我们的转化医学数据加速您的研究。
登录
或

营收
使用 Synapse 探索超过 36 万个组织的财务状况。
登录
或

科研基金(NIH)
访问超过 200 万项资助和基金信息,以提升您的研究之旅。
登录
或

投资
深入了解从初创企业到成熟企业的最新公司投资动态。
登录
或

融资
发掘融资趋势以验证和推进您的投资机会。
登录
或

生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用
