预约演示
更新于:2025-08-29

Jenkem Technology Co., Ltd.
更新于:2025-08-29
概览
标签
肿瘤
神经系统疾病
其他疾病
抗体
聚合物
mRNA
疾病领域得分
一眼洞穿机构专注的疾病领域
暂无数据
技术平台
公司药物应用最多的技术
暂无数据
靶点
公司最常开发的靶点
暂无数据
| 排名前五的药物类型 | 数量 |
|---|---|
| siRNA | 4 |
| 抗体 | 3 |
| 小分子化药 | 2 |
| ADC | 2 |
| 寡核苷酸 | 2 |
关联
16
项与 北京键凯科技股份有限公司 相关的药物靶点 |
作用机制 TOP1抑制剂 |
在研机构 |
原研机构 |
最高研发阶段临床3期 |
首次获批国家/地区- |
首次获批日期- |
靶点- |
作用机制 免疫抑制剂 |
在研机构 |
原研机构 |
在研适应症 |
非在研适应症- |
最高研发阶段临床前 |
首次获批国家/地区- |
首次获批日期- |
靶点 |
作用机制 MLPH inhibitors |
在研机构 |
原研机构 |
在研适应症 |
非在研适应症- |
最高研发阶段临床前 |
首次获批国家/地区- |
首次获批日期- |
7
项与 北京键凯科技股份有限公司 相关的临床试验NCT06586866
A Multicenter, Single-Arm, Phase 2 Study to Evaluate the Safety, Efficacy and Pharmacokinetics of JK-1201I in Triple Negative Breast Cancer Patients with Brain Metastases
This study was designed to evaluate the safety, efficacy and pharmacokinetics of JK-1201I in triple negative breast cancer patients with brain metastases.
开始日期2024-09-26 |
申办/合作机构 |
NCT06581380
A Multicenter, Randomized, Positive-Controlled, Open-label, Phase 3 Study of JK-1201I Compared with Topotecan in Patients with Relapsed Extensive Stage Small Cell Lung Cancer After Platinum-based First-line Chemotherapy
This study was designed to compare the efficacy and safety of JK-1201I with Topotecan in patients with relapsed extensive stage small cell lung cancer (ES SCLC).
开始日期2024-09-16 |
申办/合作机构 |
NCT06595186
A Multicenter, Single Arm, Open-label, Dose-escalation Phase 2 Study of JK-1201I Combined with Adjuvant Temozolomide in Patients with Newly Diagnosed Glioblastoma Multiforme (GBM) After Surgery and Concomitant Radio-chemotherapy
This study was designed to evaluate the safety, tolerability, efficacy and pharmacokinetics of JK-1201I combined with adjuvant temozolomide in patients with newly diagnosed glioblastoma multiforme after surgery and concomitant radio-chemotherapy.
开始日期2022-10-31 |
申办/合作机构 |
100 项与 北京键凯科技股份有限公司 相关的临床结果
登录后查看更多信息
0 项与 北京键凯科技股份有限公司 相关的专利(医药)
登录后查看更多信息
4
项与 北京键凯科技股份有限公司 相关的文献(医药)2025-07-01·JOURNAL OF PHARMACEUTICAL AND BIOMEDICAL ANALYSIS
Unraveling the cytotoxicity and cellular pharmacokinetic of mPEG5-NH2 polymers by UHPLC-MS/MS
Article
作者: Wang, Qingbin ; Guo, Yingxia ; Feng, Chunpeng ; Zhao, Xuan ; Yin, Lei ; Cheng, Yajie ; Tian, Jiye ; Liu, Meichen
Methoxy-polyethylene glycol amine (mPEG-NH₂), an important pharmaceutical excipient, has been extensively utilized in the field of biomedicine. To advance the development of mPEG-NH₂-related drug delivery systems, it is crucial to understand the safety profile and cellular pharmacokinetic behavior of mPEG-NH₂ polymers. In this study, a straightforward analytical assay based on ultra-high performance liquid chromatography and tandem mass spectrometry (UHPLC-MS/MS) for quantifying mPEG5-NH₂ in biological matrices was established and validated. Multiple reaction monitoring (MRM) transitions were selected for quantification of mPEG5-NH₂ (mass-to-charge ratio, m/z 252.1 → 87.7) and octaethylene glycol (OH-PEG8-OH) (mass-to-charge ratio, m/z 371.2 → 89.2). The UHPLC-MS/MS assay demonstrated excellent linearity within the concentration range of 0.01-10 μg/mL, with an R² value of 0.9996. Both intra-day and inter-day accuracies and precisions of the analytical method were within ± 9.19 %. This analytical assay was successfully applied to study the in vitro cellular toxicity and uptake behavior of mPEG5-NH₂ in MCF-7 cells. The results indicate that high doses of mPEG5-NH₂ may have potential toxicity to MCF-7 cells.
2025-06-01·Analytical and Bioanalytical Chemistry
Development and validation of a high-throughput and green ultra-performance convergence chromatography-tandem mass spectrometry assay for quantification of methoxy-polyethylene glycol propionic acid polymers
Article
作者: Feng, Chunpeng ; Zhang, JiaRui ; Yin, Lei ; Cheng, Yajie ; Zhao, Xuan ; Wang, Hecheng ; Tian, Jiye ; Deng, Yue ; Fang, Qisheng ; Wang, Qingbin ; Feng, Shuang
As a synthetic polymer, methoxy-polyethylene glycol propionic acid (M-PEG-PA) is widely used in the biomedicine field. Unraveling the pharmacokinetic behavior of M-PEG6-PA in vivo is crucial for evaluating the safety and efficiency of M-PEG-PA-related polymers or drug delivery systems. A high-throughput and green ultra-performance convergence chromatography-tandem mass spectrometry (UPC2-MS/MS) assay was firstly developed and validated for the determination of M-PEG6-PA polymers in a biological matrix. The MRM transition (mass to charge ratio, precursor ions→fragment ions) of m/z 367.2→118.9 was used to quantify M-PEG6-PA in this study. The throughput of the assay is high and the total running time for each sample was only 2 min. The linear range of the developed UPC2-MS/MS assay for quantification of M-PEG6-PA in a biological matrix is 0.05 to 30 μg/mL (R≥0.995). Intra-day and inter-day precisions for the determination of M-PEG6-PA by this analytical assay were <6.99%. The absolute recoveries and matrix effects of M-PEG6-PA ranged from 79.50 to 92.47% and 68.72 to 81.73%, respectively. The UPC2-MS/MS assay was successfully applied to quantify the concentrations of M-PEG6-PA polymers in rat plasma samples.
2025-02-01·JOURNAL OF CHROMATOGRAPHY B-ANALYTICAL TECHNOLOGIES IN THE BIOMEDICAL AND LIFE SCIENCES
Unraveling the in vivo pharmacokinetic behavior of mPEG5-NH2 polymer in rats by UHPLC-MS/MS assay
Article
作者: Wang, Qingbin ; Feng, Chunpeng ; Guo, Yingxia ; Zhao, Xuan ; Yin, Lei ; Liu, Meichen ; Tian, Jiye
As an important chemical reagent, methoxy polyethylene glycol amine (mPEG-NH2) is widely used in biomedical field. Unraveling the pharmacokinetic behavior of mPEG-NH2 polymers is essential for revealing the toxicity and efficiency of mPEG-NH2 related drug delivery systems. In this study, a simple analytical assay based on mass spectrometry (MS) was first established and validated for quantification of mPEG5-NH2 in biological matrix. The multiple reaction monitoring (MRM) transitions at m/z 252.2 (precursor ions) → 87.7 (fragment ions) and m/z 371.2 (precursor ions) → 89.2 (fragment ions) were chosen to determine mPEG5-NH2 and OH-PEG8-OH, respectively. The UHPLC-MS/MS assay showed excellent linearity over the range of 0.01-10 μg/mL. Intra-day and inter-day accuracies and precisions of the assay were all within ± 6.44 %. The analytical assay was successfully applied to reveal the in vivo pharmacokinetic behavior of mPEG5-NH2 in rats.
23
项与 北京键凯科技股份有限公司 相关的新闻(医药)2025-07-27
·药时空
一、mRNA 疫苗+肿瘤免疫疗法,催生新治疗形式1.1 mRNA 疫苗:崭露头角的全新机制疫苗RNA 药物,通过调节核酸,实现对其翻译生成的蛋白质数量与功能的调控,进而改变 病理进程,达到治疗或预防疾病的目的。在遗传学中心法则里,RNA 起着承上启下的关键作 用。从理论层面来看,对 RNA 进行调控一方面或可规避基因组改变带来的风险,另一方面也 能改善蛋白调节中常见的成药性欠佳及耐药等问题。由此可见,RNA 是一类颇为理想的药物 作用靶点。RNA 包含 mRNA(信使核糖核酸)、tRNA(转运核糖核酸)、siRNA(小干扰核糖核 酸)、miRNA(微小核糖核酸)等不同分类,其中基于 mRNA 的疗法因在新冠疫情期间被制作 成预防性疫苗而被大众熟知。 mRNA在基因表达过程中扮演承上启下的角色。DNA所携带的遗传信息经转录形成mRNA, mRNA 随后作为模板,指导核糖体进行蛋白质的合成,像是传递遗传信息的“信使”,将细胞 核内 DNA 的指令传达至细胞质中的蛋白质制造工厂。以 mRNA 作为药物可以指导细胞合成预 先设定的功能性蛋白质或抗原,从而使得“好”蛋白的表达量增加,或者引起特异性的免疫 反应。目前,mRNA 疗法以疫苗为主,已在传染性疾病、肿瘤治疗和罕见病等领域展现治疗潜 力。RNA 药物潜力无限,不同的 RNA 根据自身性质和作用机制不同,可用于不同类型适应 症的治疗。例如另一类讨论较多的核酸药物 siRNA,由 GalNAc 或其他载体进行递送,通过 dsRNA 的互补配对特异性地诱导 mRNA 降解,从而实现“坏”蛋白表达量的下调。2023 年 8 月,诺华旗下的乐可为®(英克司兰钠注射液)获中国国家药品监督管理局批准,作为饮食 的辅助疗法用于成人原发性高胆固醇血症(杂合子型家族性和非家族性)或混合型血脂异常 患者的治疗。英克司兰仅需每半年给药一次,具备显著的依从性优势。我们判断,未来 siRNA 药物仍将凭借其长效化的特点用于慢性代谢疾病的开发。疫苗是一种生物制剂,通过刺激人体免疫系统产生特异性抗体或激活免疫细胞,从而 预防特定病原体引起的疾病。传统疫苗从灭活疫苗开始,经历了减毒活疫苗、亚单位疫苗、 病毒载体疫苗和类毒素疫苗等不同技术路线的迭代,但均存在效力或毒性方面的问题,如灭 活疫苗效力较低依赖佐剂、减毒活疫苗毒性强烈但会带来较强副反应,病毒载体疫苗存在整 合到宿主基因组的潜在风险等。 mRNA 疫苗作为一种新型疫苗技术,克服了传统疫苗的多项局限,在安全性、免疫原性 和研发效率上具备综合优势。也正是由于这些优势,使 mRNA 疫苗在应对新发传染病和大规 模疫情时,展现出巨大的应用潜力,奠定了 mRNA 疫苗作为未来疫苗领域的主流技术之一的 地位。虽然初代 mRNA 疫苗的存储和运输需要超低温条件,但随着 LNP 配方和 mRNA 结构的 迭代革新,未来的 mRNA 制剂有望实现低温甚至常温条件下的稳定性,进一步便利 mRNA 疫苗 的推广应用。mRNA 疫苗本质是将修饰的单链 mRNA 组装到载体中,当前常用脂质纳米颗粒(LNP) 作为载体,形成纳米颗粒复合物。LNP 主要由可离子化脂质、DSPC、胆固醇和 PEG-脂质组 成,这些成分共同构建了一个稳定的载体系统,可以有效保护 mRNA 分子免于降解,并提高 细胞转染效率。纳米颗粒通过肌肉或者静脉注射到人体后,疫苗可被抗原呈递细胞(APCs) 摄取。细胞内的翻译机制将 mRNA 转译成相应蛋白质片段,进而激活人体免疫系统。 从免疫反应角度看,mRNA 疫苗可同时激活体液免疫和细胞免疫(MHCⅠ&Ⅱ通路)。体 液免疫中,B 细胞识别抗原后产生大量特异性抗体,形成免疫记忆;细胞免疫方面,抗原呈 递细胞激活 CD4+ T 细胞辅助激活 B 细胞和 CD8+ T 细胞,CD8+ T 细胞进一步分化为细 胞毒性 T 细胞,精准识别并杀伤表达相应抗原的肿瘤组织。作为一种肿瘤免疫疗法,免疫 记忆效应理论上可以长期存在,当再次接触相同抗原时,免疫系统可快速响应,消灭相应肿 瘤细胞,因此 mRNA 疗法从机制上适合被用作肿瘤复发转移的预防。mRNA 疫苗的产业化进程呈现出独特的路径,其在肿瘤疫苗领域的研发虽起步较早, 但预防性 mRNA 疫苗凭借新冠大流行带来的机遇实现了后发先至,推动了整个 mRNA 疫苗 技术的快速发展。 从 mRNA 疫苗的发展历史来看,1961 年 mRNA 被发现后,1971 年,mRNA 作为药物的概 念被正式提出。此后,1995 年首次使用编码癌症抗原的 mRNA 进行接种,开启了 mRNA 在肿 瘤治疗中的实验探索。2009 年,mRNA 首次直接注射用于人类癌症治疗,2012 年新抗原 mRNA 癌症疫苗的首次临床试验进一步推动了肿瘤疫苗的发展。然而,这一阶段的研究和应用主要 集中在肿瘤治疗领域,产业化进程相对缓慢。 转折点出现在 2020 年,随着新冠疫情的全球爆发,mRNA 疫苗技术在预防性疫苗领域 迎来了重大突破。Moderna 和 BioNTech/辉瑞的 mRNA 疫苗相继获准用于 COVID-19 防控,这 不仅标志着 mRNA 疫苗在预防性领域的成功应用,也极大地加速了 mRNA 疫苗的产业化进程。 2023 年,诺贝尔奖授予 mRNA 新冠疫苗研发先驱,充分彰显了 mRNA 疫苗技术的重要价值和 影响力。1.2 新冠大流行推动 mRNA 疫苗技术加速发展mRNA 疫苗在新冠大流行中实现了加速发展,并提前完成了产业化验证。2020 年 12 月, Moderna 与 BioNTech 的 mRNA 疫苗接连获批紧急使用授权(EUA)。尽管技术路径不同导致接 种剂量和储存条件有所差异,但两者都选择了 LNP 作为递送载体,并以新冠病毒的刺突糖蛋白作为编码抗原。 以销售数据来看,2021 年到 2024 年,Pfizer/BioNTech 的 mRNA 疫苗累计销售 911 亿美 金,Moderna 的 mRNA 疫苗累计销售 459 亿美金,均成为现象级的重磅产品。如以均价 20 美元/ 剂计算,两款 mRNA 新冠疫苗在四年内共销售接近 70 亿剂。巨大的销量背后是产业界在短时间内 建成了 mRNA 疫苗的大批量生产能力并克服了冷链储存运输能力的挑战,不仅验证了 mRNA 疫苗 技术的可行性,更展现了其在应对全球公共卫生危机中的巨大潜力。两款疫苗的成功为 mRNA 技 术在其他疾病领域的应用铺平了道路,预示着 mRNA 疫苗将在未来医疗健康领域扮演更加重要的 角色。Moderna 和 BioNTech 在销售模式上各有侧重,前者采用自主销售,后者选择合作销售。但 无论采用何种模式,即使 mRNA 疫苗被大量以政府协议价低价采购,在产品放量期间两家公司仍 维持了较高利润率,印证了 mRNA 疫苗整体成本的可控。我们以 2021 年第一季度 Moderna 的 Spikevax 相关数据进行生产成本的测算。该季度销售收入 17.33 亿美元,发货量 103 百万支, 单季度毛利率 88.86%,可以算出单剂均价 16.83 美元,而单剂成本仅 1.87 美元。 较低的生产成本使 mRNA 疫苗在定价上更具灵活性,对患者来说具备很高的可及性,增加 了未来成药的渗透率预期。同时,这也为 mRNA 疫苗在其他疾病领域的拓展应用提供了成本基 础,有助于推动该技术在肿瘤治疗等领域的进一步探索。开发速度方面,Moderna 和 Pfizer/BioNTech 的 mRNA 疫苗均在疫情爆发后的短短一年 内完成了从设计到获批使用的全过程,Moderna 更是在新冠病毒基因序列公布后的仅一天内完 成了疫苗序列设计,并在两个月内进行了首例人体注射。这种高效响应极致体现了 mRNA 作为一 种平台型疗法,设计之灵活与工艺开发之成熟。 由于此前并未有大量 mRNA 疗法以及 LNP 在人体内的安全性数据,因此业界对 mRNA 疫苗的 安全性仍有质疑。而在数十亿剂接种汇总的真实世界数据显示,两款 mRNA 疫苗的不良反应总体 可控,常见的不良反应主要包括注射部位疼痛、疲劳、头痛等,而上市后主要监测到为心肌炎、 心包炎、免疫和神经相关不良反应。这些反应的发生率较低,且均可通过及时的医疗干预进行有 效处理,并不会成为 mRNA 疫苗,特别是用于治疗的 mRNA 肿瘤疫苗未来推广时的障碍。1.3 IO 疗法空间广阔,肿瘤疫苗有望成为后起之秀肿瘤一直是全球范围内导致死亡的最主要原因之一,临床对有效治疗手段的需求远未得到 满足。据 Frost&Sullivan 数据显示,全球癌症药物市场在 2018 年到 2023 年间,从 1,281 亿美 元增长至 2,289 亿美元,年复合增长率达到 12.3%。预计从 2023 年到 2028 年,该市场将以 9.5% 的年复合增长率继续扩大;2028 年至 2032 年,增长率将稳定在 7.8%,最终在 2032 年达到 4,868 亿美元。这表明肿瘤药物市场不仅规模庞大,且增长趋势稳定。 尽管在部分癌种治疗上取得了突破性的进展,但仍有很多恶性肿瘤五年生存率在极低水平, 临床需求远远未得到满足。这一现象反映出不同肿瘤类型在治疗难度和预后方面的差异,也凸显 出对于低生存率癌种开发更有效治疗手段的迫切性。肿瘤免疫(Immuno-Oncology,IO)疗法作为一种创新的癌症治疗方法,通过利用患者自身 的免疫系统来清除肿瘤细胞。免疫系统具备区分“自身”与“非自身”的关键能力。在正常情况下, 健康细胞产生的分子不会引发免疫反应。然而,当细菌和病毒等病原体出现时,其与健康细胞明 显不同的分子特征(即抗原)会激发免疫系统的反应。肿瘤细胞由于快速增殖的需要,会过多地 生产健康细胞相同分子(肿瘤相关抗原,TAA),或突变出某些特异性分子,能够引起免疫反应(新 抗原),但这种反应的强度通常不足以清除肿瘤组织。 为解决这一问题,目前有多种免疫疗法正在开发和应用。其中包含解除免疫抑制的“松刹 车”以及增强免疫活性的“踩油门”两条路径。例如,免疫检查点抑制剂(Immune Checkpoint Inhibitors,ICIs)可以解除肿瘤细胞对 T 细胞的抑制,使 T 细胞重新识别并攻击肿瘤;嵌合抗 原受体 T 细胞疗法(CAR-T 细胞疗法)通过体外基因工程改造患者的 T 细胞,使其表达能够特异 性识别肿瘤细胞表面抗原的嵌合抗原受体,然后将这些改造后的 T 细胞回输到患者体内,从而精 准、高效地消灭肿瘤细胞。以 PD-1/PD-L1 抗体为代表的 ICIs 展现出巨大的市场潜力和临床价值。自首款 PD-1 抑 制剂上市以来,该领域重磅产品频出,多款 PD-1 单抗已成为重磅炸弹,在全球范围内广泛应 用。根据 Precedence Research 的预测数据,全球 ICIs 药物市场规模呈现稳步增长趋势,预 计到 2034 年将达到 2300 亿美元。 ICIs 药物作为肿瘤免疫疗法的基石,能够广泛覆盖多种癌种,这是其成为大品种的关键 保障。以帕博利珠单抗(Keytruda,下文称 K 药)为例,K 药布局了包括乳腺癌、肺癌、头颈鳞 癌、胃癌、肝癌、MSI-H/dMMR 肿瘤等多个大适应症在内,单药以及各种联用,涵盖围术期、一 线、后线疗法的适应症矩阵,且仍在探索新适应症和新给药方式。广泛的适应症布局给 K 药带来 了巨大的销售额,2023 年,K 药依靠 250.11 亿美元销售收入彻底击败修美乐,成为新一代全球 “药王”。2024 年,K 药继续霸榜,为默沙东创收 294.82 亿美元。IO 治疗尽管已存在多种成熟疗法,但它们各自面临的问题限制了临床上进一步地推广应 用。例如,ICIs 在部分瘤种响应率低,对患者肿瘤突变负荷和免疫细胞浸润要求高;CAR-T 生 产流程复杂,成本高昂,且在实体瘤中尚需验证;TCE 疗法已展现治疗潜力,但仍有脱靶、T 细 胞耗竭以及引发细胞因子风暴等安全性问题。 mRNA 肿瘤疫苗可针对多种肿瘤进行设计,具个性化治疗潜力,安全性佳,成本较低,且 可以实现对肿瘤的长期免疫监视和控制。我们认为 mRNA 肿瘤疫苗有望弥补现有疗法缺陷,成为 IO 领域的新治疗范式,为肿瘤患者提供更有效、经济、安全的治疗选择。二、mRNA 肿瘤疫苗:用药策略趋于成熟,彰显多重潜力2.1 mRNA 肿瘤疫苗:治疗性疫苗的先行军在传统认知中,疫苗是被用来提前接种,从而在流行季预防传染性疾病的医疗产品。而从 原理上看,凡是通过提升抗原暴露量,“教会”免疫系统识别异己的产品都可以被称作疫苗。肿 瘤疫苗作为一种新兴的免疫治疗方法,通过暴露肿瘤特异性抗原,使免疫系统建立对肿瘤的识别 能力,从而实现对肿瘤的免疫清除。虽然并不起到预防性的功能,但仍可称为疫苗。 以 mRNA 疫苗为例,肿瘤疫苗的作用机制可分为以下几个关键步骤: 1. 药物设计阶段,研发人员会精心选择一组具备肿瘤特异性和良好免疫原性的抗原,根据在健 康细胞和癌细胞的表达情况可被分为新抗原(neoantigen)、肿瘤相关抗原(Tumor - Associated Antigen,TAA)以及肿瘤特异性抗原(Tumor - Specific Antigen,TSA)。这些抗原的 mRNA 序 列经过优化后,被接入提前设计好的 mRNA 框架中 2. 合成的 mRNA 装载在 LNP 中,形成稳定的药物制剂。通过肌肉或静脉注射,mRNA 疫苗被递 送至患者体内。 3. 在体内,mRNA 疫苗被抗原呈递细胞(APCs)摄取,这些细胞在脂质体的保护下高效表达肿 瘤抗原,与 MHC 结合后展示在细胞表面。 4. DC 细胞等 APCs 重定位到外周淋巴器官,激活 CD4+和 CD8+T 细胞增殖分化,从而建立起肿 瘤特异性免疫。细胞毒性 T 细胞会对癌细胞进行特异性杀伤,分化的记忆 T 细胞可以长期存活。在肿瘤疫苗开发的过程中,除了 mRNA 疫苗外,也同时存在多肽疫苗和 DC 细胞疫苗两种技 术路径。多肽疫苗生产工艺成熟,但免疫原性较弱,往往需要依赖佐剂增强免疫反应; DC 细胞 疫苗需经过体外细胞培养再回输患者体内,操作流程复杂,带来高昂的成本,极大限制了疗法的 可及性。而 mRNA 疫苗通过 RNA 序列携带抗原信息,不依赖佐剂,生产便捷灵活,成本较低。此 外,基于 mRNA 路径的肿瘤疫苗还存在以下独特优势。 优势 1 :mRNA 肿瘤疫苗分为个性化疫苗与通用性疫苗两种设计路径,个性化疫苗可实现精 准医疗,通用疫苗有望实现现货化。个性化疫苗是一种定制化的产品,根据每个人的突变分 布和 HLA 表型选择更高免疫原性的肿瘤新抗原组合,能够实现精准医疗,为每一个人带来 更高的免疫刺激;而通用疫苗通过挑选患者人群中常见的 TAA/TSA 组合,即可以在大部分 患者中应用,又能做到规模化生产和配送,并随时响应患者治疗需求,实现 “现货化”治 疗。 优势 2:多价设计有望解决肿瘤异质性和耐药。异质性是恶性肿瘤的特征之一,异质性的存 在,使不同亚群对药物的敏感程度不同,长期用药会导致耐药从而进展。而 mRNA 肿瘤疫苗 可同时装载数十种抗原 RNA,覆盖不同亚群的抗原表位,从而有望通过覆盖范围的提升突破 异质性。 优势 3:同时激活 CD4+与 CD8+ T 细胞,能够形成长期免疫记忆。mRNA 疫苗转染 APCs 后, 可同时通过 MHC I 和 MHC II 同时激活 CD4⁺ 与 CD8⁺ T 细胞,从而实现特异性肿瘤杀伤以及 T 细胞的长期存活。2.2 通用疫苗有望率先实现现货化通用肿瘤疫苗,也被称作共享肿瘤疫苗,其设计核心在于选取在不同患者中普遍表达的 TAA 或 TSA,而非针对每位患者进行个体化定制,因此可预先大规模生产并储存,实现现货化(offshelf)销售。这样的疗法可以减少患者的等待时间,提高治疗的及时性;此外规模化生产带来 较低的成本和定价,从而具有较强可及性。 目前,通用 mRNA 肿瘤疫苗主要基于两种设计思路。一种是按照瘤种来设计,针对特定类 型肿瘤的特异性抗原开发疫苗,如 BNT111 为黑色素瘤设计、BNT116 专为非小细胞肺癌设计等; 另一种是选取泛癌种表达的 TAA,以此寻求在多种适应症上的广泛应用布局,如 mRNA-4359 选择 IDO/PD-L1。多价通用疫苗可通过至少一种抗原引起特异性免疫反应,从而进行疾病控制。以 BNT116 为例,该疫苗包含 6 种编码肿瘤相关抗原(TAA)的 mRNA。2024 年 SITC 公布的 Ⅰ/Ⅱ 期试 验数据显示,BNT116 在联合西米普利单抗治疗 PD-L1 表达阳性的 PD-1 经治非小细胞肺癌患者 试验中,疾病控制率(DCR)达到 80%(16/20),中位无进展生存期(PFS)为 5.5 个月。免疫原性分析显示部分患者对疫苗编码的多种抗原产生免疫反应,其中一例患者仅对两种抗原产生免 疫响应,也可起到疾病控制的效果。2.3 个性化疫苗有望突破肿瘤异质性,AI 助力开发提速个性化 mRNA 肿瘤疫苗通过针对每位患者独特的癌症新抗原进行定制化设计,为每一位肿 瘤患者提供精准解决方案,这种设计思路有望克服肿瘤异质性带来的治疗障碍,且抗原选择算法 对不同癌症均有效,在于能够在不同肿瘤中激发高强度免疫应答。 目前,国际上进展显著的个性化 mRNA 肿瘤疫苗包括 BNT122 和 mRNA-4157,二者目前均 选择与 PD-(L)1 单抗联合使用,适应症集中于辅助疗法,同时探索一线/末线治疗的可能性。 BNT122 的临床试验覆盖多种适应症,包括晚期黑色素瘤、胰腺导管腺癌(PDAC)、肌肉浸润性尿 路上皮癌(MIUC)等。mRNA-4157 聚焦辅助疗法,其中可切除的高风险黑色素瘤已推进到 III 期, 有望成为首个获批商业化的 mRNA 肿瘤疫苗。个性化疫苗可在不同肿瘤中激活免疫反应,且激活的 T 细胞可浸润肿瘤组织。2025 年 1 月,BNT122 单药或联用阿替利珠单抗的 I 期临床试验结果在 nature medicine 发表,数据显 示在包括结直肠癌、三阴性乳腺癌、黑色素瘤、非小细胞肺癌等复发晚期病人中有 71% 的患者 对个性化疫苗产生响应,且在肿瘤病灶中可检测到新抗原诱导的从头合成的 T 细胞,证实了个 性化疫苗良好的免疫激活效果和 T 细胞浸润能力。个性化疫苗的设计和生产流程可整合到肿瘤诊疗流程中,但患者等待时间仍然较长,亟需 压缩。以辅助用药为例,首先患者为了确诊肿瘤/手术切除会取出部分肿瘤组织并测序,疫苗生 产企业在获取测序结果后可推进新抗原预测、设计个性化 mRNA 序列以及进行小批量生产生产 疫苗。与此同时,患者可进行肿瘤的切除或化疗等前线处理,在进行一定恢复后个性化 mRNA 疫 苗也已完成生产,可以进行接种。根据 Moderna 披露,这一设计-生产周期约为 6 周,虽然可以 与癌症病人的前线治疗同时进行,但对一些晚期患者来说,有限的无复发生存时间经不起耽搁。 可喜的是,AI 技术的突破有望大幅压缩等待时间。AI 技术正在革新 mRNA 肿瘤疫苗的研发流程,显著提升开发效率并助力突破技术瓶颈。 2025 年初,美国星际之门计划公司宣布将投资 5000 亿美金建设人工智能基础设施,其投资方之 一甲骨文集团的 CEO 拉里・埃里森在发布会上表示,该计划落地后将助力 AI 增效,将个性化 mRNA 肿瘤疫苗的开发时间压缩至 48 小时。这凸显了 AI 在疫苗研发中的巨大潜力。 头部 mRNA 疫苗公司早已将 AI 技术融入研发流程。Moderna 自 2016 年起便引入 AI 进行个 性化 mRNA 肿瘤疫苗序列设计,并于 2023 年与 OpenAI 全面加强合作。BioNTech 自 2011 年开始 利用计算机设计 mRNA 疫苗,2017 年采用程序选择新抗原,2023 年收购 InstaDeep 进一步深化 AI 赋能。这些举措体现了 AI 在加速 mRNA 肿瘤疫苗研发方面的关键作用。AI 可解决 mRNA 药物开发痛点。首先,核酸存储的信息量巨大,在序列设计过程中需要排 除大量干扰,且根据 MHC 结合强度/数目,病原体序列相似度等多维度标准来选取最优抗原组合, 这一过程对计算效率和准确度要求极高;其次, mRNA 在成药前需经过复杂的化学修饰与密码子 优化等步骤,不同的优化方式会进一步带来 mRNA 的翻译效率、二级结构、稳定性、对应多肽免 疫原性的变化,对序列理解的要求非常复杂。此外,由于密码子存在简并性,通过穷举并排序非 常困难。 而 AI 技术的引入,使得解决这些复杂问题成为可能。以 Zhang, H.等人发表在 nature 的 一种 mRNA 新冠疫苗的结构优化算法为例,1273 个氨基酸残基的新冠刺突蛋白对应 3822 个核苷 酸,如果对所有序列优化可能进行穷举需要 2.4 x 10632种可能性,需要约 10617亿年进行计算, 而使用动态算法可将整个过程压缩至 11 分钟,大幅提升序列优化效率。AI 在核酸药物优化方面的应用具有天然优势。核酸领域拥有 GenBank、RefSeq 等高质量 数据库,这些数据库为 AI 模型的学习训练提供了丰富的数据来源。同时,核酸表征方法的多样 性使得核酸数据能够被转化为适配 AI 算法的向量形式,且在转化过程中原始信息得以最大程 度保留。因此, AI 技术在 mRNA 肿瘤疫苗研发中的应用,为个性化肿瘤疫苗的设计开发提供了 强大工具,有望大幅压缩患者等待时间。2.4 ICIs + mRNA 有潜力成为未来标准疗法如前文所述,ICIs 作为肿瘤免疫治疗的基石,在多种癌症治疗中已取得显著成果,但在 部分瘤种中的响应率仍有待提高。例如,PD-(L)1 抗体类药物在部分瘤种中,ORR(客观缓解 率)仅为 10%-30%,而在胶质母细胞瘤、卵巢癌等瘤种中甚至更低。ICIs 的疗效与患者肿瘤的 突变负荷密切相关,对于突变负荷较低的肿瘤类型,不同 ICIs 的疗效均有限。这可能与 ICIs 在 肿瘤免疫中仅扮演“踩刹车”角色有关,突变负荷较低、肿瘤微环境较强或者自身免疫系统较弱 的患者难以在 ICIs 治疗中获益。因此为了提高 ICIs 的适应症覆盖,相关企业均广泛探索联用可 能。 mRNA 肿瘤疫苗从机制上与 ICIs 互相配合,可实现 1+1>2。mRNA 肿瘤疫苗可以筛选免疫原 性较高的抗原,“教会”免疫系统识别突变负荷较低的肿瘤,诱导从头合成的免疫反应并增强已 有免疫反应,从而起到“加油门”的角色,与“踩刹车”配合,解决 ICIs 响应率不足的问题。 同时,ICIs 可以接触肿瘤组织的免疫抑制,增强疫苗诱导的特异性 T 细胞的活性。mRNA 肿瘤疫 苗与 ICIs 的联合应用通过机制互补和协同增效,有望克服 ICIs 单独使用的局限性,提高肿 瘤治疗的响应率,进而转化为患者生存率。ICIs + mRNA 已在不同产品上获得概念验证,有潜力成为未来标准疗法。我们对三项代表 性临床试验进行梳理分析,可以明显看到 ICIs + mRNA 在辅助疗法和晚期患者中的优异表现。2.4.1 KEYNOTE-942 研究:mRNA-4157 联用 K 药用于黑色素瘤辅助疗法,可显著延长复发转移, 且增强 PD-L1 阴性患者获益。mRNA 肿瘤疫苗适合用作辅助治疗,防止肿瘤复发转移。辅助治疗通常是在可手术的病 人手术后给予的治疗,用来消灭体内残余的癌细胞,并降低肿瘤复发或向其他部位播散的可 能性。在手术后的肿瘤环境中,肿瘤负荷较小,微环境被破坏,免疫系统相对健康,本身适 合肿瘤免疫的激活。然而,部分瘤种在经历辅助治疗后复发率仍然较高,例如胰腺导管腺癌 (PDAC)5 年复发率接近 70%,即使在 ICIs 高响应的黑色素瘤,在接受 PD-1 单抗辅助治疗 后 18 个月仍有约 40%患者复发。而肿瘤疫苗作为可以激活免疫记忆的新疗法,诱导生成的 记忆性免疫反应或可应用到长期复发的预防中。KEYNOTE-942 是 Moderna 与 MSD 合作开展的一项开放标签、随机、2b 期辅助研究,研 究共纳入 157 例 IIIB - IV 期皮肤黑色素瘤患者,使用组织样本进行 NGS 测序。联合治疗 组采用 mRNA-4157 + 帕博利珠单抗联合治疗。mRNA-4157 剂量为 1mg,肌肉注射,每 3 周1 次,最多 9 剂;帕博利珠单抗最多 18 个周期 。对照治疗组采用帕博利珠单抗单药治疗, 最多 18 个周期 。实验的主要终点是无复发生存期(RFS),次要终点为无远处转移生存期 (DMFS)、安全性以及耐受性 。2024 年,该实验的三年随访数据更新在 ASCO 公布。与单独使用 K 药相比,mRNA-4157 (V940) 联合 K 药辅助治疗继续显示出无复发生存期 (RFS) 的临床意义和持久改善,。 联合用药组将复发或死亡风险降低了 49%(HR= 0.510),将发生远处转移或死亡的风险降低 了 62%(HR= 0.384)。由此,mRNA 肿瘤疫苗首次在随机临床试验中证实其参与癌症治疗的 有效性,证明 mRNA+ICIs 可改善肿瘤预后,且提供了长期随访数据。值得注意的是,在 KEYNOTE-942 18 个月数据的亚组分析中,联合疗法显著提升了 PDL1 表达阴性的患者的获益,PD-L1 阴性的患者接受联合治疗后 18 个月复发率仅 23%,对比 K 药单药获益显著,HR 低至 0.162。这说明 mRNA 肿瘤疫苗对 PD-1 单抗的增敏效应不依赖 PD-L1 的表达情况,有望通过联用进一步扩展 PD-1 的适应症范围。2.4.2 BNT122 用于 PDAC 辅助疗法的一期临床研究:在冷肿瘤中诱导免疫反应,并展现激发长期 记忆能力。胰腺癌是所有常见恶性实体瘤中预后最差的,5 年总生存率约为 10%。据估计,到 2020 年美国将有 57,600 人被诊断为胰腺癌,47,050 人将死于胰腺癌,成为第三大癌症死亡原因。 大约 95%的胰腺癌是外分泌细胞肿瘤,最常见的是胰腺导管腺癌(PDAC),即使进行了手术切 除,但仍有近 90% 的患者在中位 7-9 个月内复发。PDAC 难治性的主要原因在于其免疫微 环境中的低免疫细胞浸润、强免疫抑制、高异质性和高基质密度特征,使得 PDAC 对大多数 靶向治疗和免疫治疗的反应不佳,而被定义为“冷肿瘤”。在 2024 年 NCCN 与 CSCO 推荐的辅 助疗法中,未纳入任何一项包含 ICI 的疗法。研究人员发现,虽然 PDAC 整体突变负荷低,但仍含有足够数量新抗原,可以通过定制 肿瘤新抗原组合,使用 mRNA 肿瘤疫苗以激活免疫反应。一项由纪念斯隆凯特琳癌症中心 (MSKCC)研究者发起的单中心 I 期临床试验,纳入 16 PDAC 患者接受研究性治疗性癌 症疫苗 BNT122,联用阿替利珠单抗与 mFOLFIRINOX 化疗方案。该实验中所有患者都接种了个性化设计的,包含至少 7 种,至多 20 种肿瘤新抗原的 个性化疫苗。在 18 个月随访时,50%患者(n=8)对至少一种肿瘤新抗原产生响应。MSKCC 随 后对全部患者进行了持续随访,并在 2025 年 3 月份将中位随访时间 3.2 年的跟踪数据分析 发表于 nature 上。研究发现,对疫苗的响应与长期获益有关。在对疫苗产生反应的 8 患 者中,有 6 在随访期间没有看到癌症复发,而无响应患者中复发 7 人。在产生反应的 8 患者中,有 4 人仅对个性化疫苗中的一种抗原产生响应,但即使单抗原的响应仍可转换为 临床获益。BNT122 激活的 T 细胞可以实现长期记忆,并长期维持效应功能。在患者样本分析中同 时发现,在疫苗响应人群中,给药后长达 3 年内仍可检测到超过 80%的疫苗诱导的新抗原特 异性 T 细胞。与无反应者相比,这些患者的中位无复发生存期延长。疫苗诱导的 T 细胞克隆 预估中位寿命为 7.7 年,其中约 20%的克隆具有数十年的寿命,甚至比宿主活得更久。这一 趋势可能转化为潜在的复发预防获益。此外,患者是否对 PD-L1 抗体产生反应与 RFS 无关, 证明肿瘤疫苗是联合疗法中的关键角色,而阿替利珠单抗仅起辅助角色。2.4.3 Lipo-MERIT 研究:BNT111 联用 PD-1 单抗治疗复发晚期黑色素瘤,在晚期高风险疾病、 ICI 经治人群中取得疗效。BNT111 是基于 BioNTech 的 FixVac 平台设计的一款通用 mRNA 肿瘤疫苗,该疫苗编 码四种常见的黑色素瘤相关抗原(TAA),包括 NY-ESO-1、MAGE-A3、酪氨酸酶和 TPTE。LipoMERIT 研究是一项剂量递增的 I 期临床试验,纳入了在四种 TAA 中至少表达其中一个的 IIIb-IV 期黑色素瘤患者,并且在入组患者中, 98% 的患者既往接受过至少一种 ICIs 治 疗。患者将接受 8 剂疫苗接种,同时部分联用 PD-1 单抗。研究设计旨在评估 BNT111 单药 及联合 PD-1 单抗的安全性和疗效。研究结果显示,BNT111 联合 PD-1 单抗能够显著增强 PD-1 经治人群中的免疫反应。 在接受肿瘤疫苗接种的患者中,75%以上的患者对至少一种抗原发生免疫反应,且体外分析 发现疫苗诱导的免疫反应以 CD8+ T 细胞为主。联合治疗将患者对 TAA 的免疫响应率从 64% 提升至 92%,增强了单药的免疫反应。联合疗法的获益可能与缓解率提升有关,在联合用药 组(FixVac+aPD1)中,17 例患者中有 6 例 PR,ORR 47%;而单药组中这一比例为 16%。通过联合 PD-1 单抗,BNT111 不仅能够增强免疫反应,还能提高患者的生存获益。 因此 BioNTech 将联合疗法推进 II 期,并于 2024 年 7 月宣布该 II 期临床试验达到了主要 疗效结果指标,表明在该适应症和治疗环境中,与历史对照相比,BNT111 联合西米普利单 抗治疗的患者的 ORR 具有统计学意义改善,研究中单药治疗组的 ORR 与抗 PD-(L)1 或 抗 CTLA-4 治疗的历史对照一致。 ICIs + mRNA 联用:重塑肿瘤免疫治疗格局。ICIs(免疫检查点抑制剂)与 mRNA 肿 瘤疫苗的联合应用,正在展现出对现有肿瘤治疗方法的显著增益。这种联合疗法在多种癌症 类型(包括 PDAC 等冷肿瘤)中表现出提升响应率、延长无复发生存期(RFS)的能力,实 现长期免疫记忆,且不受患者 PD-L1 表达水平的影响。因此,ICIs + mRNA 联用有望成为 下一代肿瘤免疫疗法的核心框架,具备改写多个癌种标准治疗范式的潜力。 正是由于认识到这种趋势,中外明星 biotech 企业均积极开展下一代 ICIs 与 mRNA 联 用探索。 作为 mRNA 疗法龙头,BioNTech 除了积极推进自身肿瘤疫苗管线的开发外,还积极拥 抱中国创新药资产,通过收购普米斯获得 PD-L1xVEGF 双抗,以及引入了映恩生物与宜联生物的多款 ADC 管线。这一系列战略布局,构建起“mRNA+下一代 ICIs+ADC”的治疗矩阵,提供 探索进一步联合用药的可能性。 无独有偶,康方生物于 2025 年 4 月 6 日在 Clinicaltrials.gov 注册了一项 IIT 临床 试验,计划入组 60 例可手术胰腺癌患者,研究个性化 mRNA 疫苗 AK154 单药或联合 PD1/CTLA-4 双抗、PD-1/VEGF 双抗以及 mFOLFIRINOX 化疗辅助治疗 PDAC 的疗效。该试验沿用 BNT122 在 PDAC 辅助疗法的实验设计,但将 PD-1 单抗升级为双抗,彰显出免疫双抗龙头企 业对下一代 ICIs+mRNA 联用策略的看好和战略布局。mRNA 肿瘤疫苗安全性优异,不会给接受联合疗法的患者带来新增严重不良反应。例如 在 BNT122 联用或不联用阿替利珠单抗治疗晚期实体瘤的 Ib 期试验中,单药组中 10%患者出 现 3 级不良事件,未观察到 4 级和 5 级 TRAE。联合用药组中,19.1%患者出现 3 级以上 TRAEs, 免疫介导的 AE 的严重程度、频率和临床性质与阿替利珠单药治疗经验基本一致。 良好的安全性使得 mRNA 肿瘤疫苗有机会具备探索更多疗法联用可能。目前势头正盛 的 IO+ADC,IO+化疗,CAR-T、TCE 等肿瘤免疫单药疗法,在原理上都可与 mRNA 肿瘤疫苗进 行联用,提高肿瘤免疫的活性和靶向性。同时,mRNA 肿瘤疫苗并不会给患者带来新增的、 mRNA 疫苗相关的不良反应,因此有理由相信未来会开展大量联用探索,并为肿瘤患者提供 更多治疗选择。2.5 联用 ICIs and beyond,百亿美金潜力待释放综上所述,我们认为 mRNA 肿瘤疫苗可以通过三条路径逐步打开市场空间:1)联用 ICIs 并进入 ICIs 目前覆盖的适应症领域;2)拓展 ICIs 的应用范畴,进入过往响应率较低的适 应症;3)凭借高度安全性探索更多药物联用潜力,如 ADC、CAR-T 等。 因此,我们以 ICIs 药物的覆盖人群为锚测算 mRNA 肿瘤疫苗市场。1. 根据 Precedence Research 预测,ICIs 2024 年市场规模预计 502.8 亿美金,其 中 PD-(L)1 单抗占据绝大多数。主要有 K 药(帕博利珠单抗,2024 年销售额 295 亿美金),O 药(纳武利尤单抗,2024 年销售额 101 亿美金),T 药(阿替利 珠单抗,2024 年销售额 41 亿美金)等;2. 欧美市场是创新药品的核心市场,根据多家 MNC 的欧美收入占比,我们假设欧美 市场占全球市场的 75%;3. 根据测算,我们假设 ICIs 在欧美市场年费用平均 15 万美元,因此如按每位患者 均足量使用全年药物来算,欧美每年使用 ICIs 患者约 25.14 万人。实际使用人 群应该远大于这一数字,但我们仍按此数字作为计算 mRNA 肿瘤疫苗潜在患者人 群的基数;4. 根据 BioNTech 披露,目前 PD-(L)1 已获批/未获批适应症在欧美的年新发病人群 分别约为 150 万/140 万人,即潜在可开拓适应症空间大于 90%。因此,我们从两 个维度交叉对 mRNA 肿瘤疫苗与 ICIs 联用情况进行情景分析,其中 mRNA 肿瘤疫 苗联用策略占 ICIs 疗法比例按悲观/中性/乐观分别给予 40%/70%/100%,通过联 用使 ICIs 拓展适应症比例按悲观/中性/乐观分别给予 100%/140%/180%。经过较 差分析,我们选择 40%/98%/100%作为未来 mRNA 肿瘤疫苗联用占 2024 年 ICIs 患 者人数百分比的悲观/中性/乐观数据;5. 根据产业调研与测算,通用型疫苗由于可以规模化生产配送与长期存储,其生产 成本可能与mRNA新冠疫苗维持在相同量级;而个性化疫苗由于需要定制化生产, 其中的测序、抗原预测、小批量生产与质控、小批量配送等环节产生的额外费用使得个性化疫苗的生产成本将高出几个数量级。反应到价格端,我们按照每疗程 使用 9 剂计算,假设通用化疫苗单剂价格 5000 美金左右,年费用 4.5 万美金; 个性化疫苗单剂价格 3 万美金,年费用 27 亿美金附近;6. 根据通用疫苗与个性化疫苗的特性,我们针对使用肿瘤疫苗患者中通用疫苗与个 性化疫苗的占比进行了情景分析,我们推断由于存在价格优势,通用疫苗可能相 对个性化疫苗获取更高的渗透率。其中: 情景 1:通用性疫苗使用量占 80%,个性化疫苗使用量占 20%; 情景 2:通用性疫苗使用量占 65%,个性化疫苗使用量占 35%; 情景 3:通用性疫苗使用量占 50%,个性化疫苗使用量占 50%;根据测算,mRNA 肿瘤疫苗的市场空间在 120.67 亿到 950.29 亿美元之间,其中中性情 形下市场规模约在 406.51 亿美金。因此,仅在 ICIs 联用下 mRNA 肿瘤疫苗便具备数百亿美 金市场潜力,将带来多款重磅炸弹。三、海外管线研究:龙头公司策略有别,将迎密集催化BioNTech、Moderna 和 Curevac 是新冠疫情时倍受市场广泛关注的 mRNA 龙头,他们 正是因为早在多年前便开始探索肿瘤疫苗,从而具备了 mRNA 疫苗的药物设计和递送技术等 研发平台,因此得以快速切入新冠预防性疫苗赛道。虽然疫情后在经营上做出了不同选择, 但在 mRNA 肿瘤疫苗领域三家公司管线均值得关注。3.1 BioNTech:与下一代 ICI 联用值得期待BioNTech 目前多元布局肿瘤疗法,拥有下一代 ICI PD-L1/VEGF 双抗,以及多个靶点 的创新 ADC,未来有望与肿瘤疫苗联用,率先展示肿瘤疫苗的联用潜力。 公司的新冠疫苗因与辉瑞、复星医药等合作而被熟知,其中在中国,BioNTech 与复星 医药成立合资公司(各持股 50%),复星投入生产设施(年产能达 10 亿剂),BioNTech 则贡献技术转移。尽管疫苗最终未在中国内地获批,但合作奠定了 BioNTech 与中国企业的信任 基础,并为其后续布局提供了本地化经验。 在肿瘤领域,疫情后拥有大量现金的 BioNTech 不再拘泥于 mRNA 疫苗这一细分类别, 而是进行多元化布局,特别是引入多项中国资产,借助中国创新药企业的研发效率快速扩充 管线。其中 ADC 药物方面首先与映恩生物合作引进靶向 HER2 和 Trop2 的 ADC(DB-1303、DB1305),总金额超 15 亿美元,并与宜联生物合作开发 HER3 ADC,总额超 10 亿美元。抗体方 面,BioNTech 不但继续深化与 Genmab 的合作,更是先后通过普米斯的收购获得了下一代 ICIs 基石药物 PD-L1/VEGF 双抗,以及与 OncoC4(昂科免疫)达成合作协议,引进后者的新 一代 CTLA-4 抗体 ONC-392。 而 mRNA 肿瘤疫苗方面,公司同时注重个性化和通用疫苗的研发。其中个性化疫苗研 发以 BNT122(autogene cevumeran)为核心,其目前已证实在胰腺导管腺癌(PDAC)辅助和 黑色素瘤等多个瘤种中诱导新抗原特异性 T 细胞,并展现出可持续的免疫反应。通用疫苗方 面布局同样丰富,涵盖了多种癌症类型。BNT111 是针对黑色素瘤设计的通用疫苗,编码四种 常见的黑色素瘤相关抗原(TAA),在晚期黑色素瘤患者中,BNT111 与西米普利单抗联合使 用,已公告在一项 II 期临床顶线数据中实现了 ORR 改善。此外还有针对 HPV16 相关头颈癌 设计的 BNT113、针对非小细胞肺癌(NSCLC)设计的 BNT116 在临床探索中。3.2 Moderna:个性化疫苗将迎突破Moderna 在疫情后仍然聚焦 mRNA 技术,并逐渐扩大了在预防性疫苗上的研发投入和管 线数量,在肿瘤疫苗领域的管线数量不断减少,目前重点聚焦期个性化疫苗 mRNA-4157,在 多个癌种联用 K 药开展辅助疗法实验。例如前文介绍过与默沙东合作的 KEYNOTE-942 研究, mRNA-4157 联合帕博利珠单抗在可切除的高风险黑色素瘤辅助治疗中,三年随访数据显示 联用疗法对比帕博利珠单抗单药显著延长无复发生存期(RFS)。mRNA-4157 有望于 2027 年 起进入商业化阶段,成为首个上市的 mRNA 肿瘤疫苗。 Moderna 的通用疫苗尝试布局泛瘤种策略,但 2025 年 JPM 上披露已缩减至仅一款。 mRNA-4359 选择了普遍表达的两种 TAA IDO (吲哚胺 2,3 - 双加氧酶)与 PD-L1 组合用于 晚期或转移性皮肤黑色素瘤、非小细胞肺癌(NSCLC)等的治疗。 Moderna 致力于探索基于 mRNA 的更多形式肿瘤疗法,尽管其四价 KRAS 疫苗(mRNA5671)和 OX40L/IL-23/IL-36 γ 免疫调节疗法(mRNA-2752)等项目已暂停开发,但公司仍 在推进体内 CAR-M、细胞疗法增强剂等临床前项目,致力于通过技术创新拓展 mRNA 在肿瘤 治疗中的应用边界。3.3 CureVac:重新聚焦肿瘤,开发通用性疫苗CureVac 作为 mRNA 技术领域的早期探索者,在新冠疫情期间经历了多次合作与研发的 波折,例如与拜耳合作的第一代新冠疫苗失败,与第二代新冠疫苗葛兰素史克 (GSK)的开发 不及预期等。公司在 2024 年开启重组计划,以 4 亿欧元首付款、高达 10.5 亿欧元的价格将 流感疫苗和新冠疫苗全球权益剥离给 GSK,同时计划裁员 30%。 随着 CureVac 研发重回正轨,公司计划在其胶质母细胞瘤的 Ib 期临床试验进行给药, 重启肿瘤疫苗的研发。目前,CureVac 进展最快的项目是通用疫苗 CVGBM,该疫苗编码四种 胶质母细胞瘤相关肿瘤相关抗原(TAA)的八个抗原。已公布的 I 期临床试验数据显示,在 part A 分组中,77% 的患者对疫苗产生了免疫反应,其中八成以上的患者 T 细胞为抗原 诱导的从头合成。这表明 CVGBM 在激发免疫反应方面具有显著效果。公司另外一款治疗鳞 状非小细胞肺癌的通用疫苗预计将于 H2 2025 推进临床,进一步充实公司通用疫苗管线。3.4 海外公司 mRNA 肿瘤疫苗管线2025 催化剂梳理2025 年通用型和个性化疫苗均有多款数据流出,将成为 mRNA 肿瘤疫苗领域的重要 节点。其中包含 mRNA 肿瘤疫苗领域首个Ⅲ期有效性数据,结直肠癌、非小细胞肺癌等适应 症的首个Ⅱ期数据等。 而展望 2026 年及以后,我们认为随着 BNT112 的 PDAC 数据、mRNA-4157 的首个适 应症获批,以及更多基于 mRNA 的治疗性疫苗进入临床,mRNA 肿瘤疫苗赛道将逐渐升温, 有望在不同瘤种中获得更多的临床数据验证,并跨过从 0 到 1 的边界。四、国内先行者已入局,成熟平台或可赶超国内 mRNA 肿瘤疫苗领域呈现出蓬勃发展的态势,上市公司和初创企业均积极布局, 并开展了多项合作。尽管整体管线进展相较于 BioNTech 和 Moderna 等全球龙头企业稍显 缓慢,但我们对国内公司实现赶超充满信心,主要基于以下考量: 首先,mRNA 药物研发具有高度工程化和平台化的特点。一旦技术取得突破,将能够 迅速拓展多项应用。由于 mRNA 药物的特点,除编码区外,mRNA 的修饰技术、元件、递送系 统等均可通用,因此 mRNA 药物相关的技术储备和 know-how 可以跨越不同适应症应用。例 如,Moderna 的个性化 mRNA 肿瘤疫苗使用的 mRNA 修饰技术也曾用于其新冠疫苗 mRNA-1273, 并在新冠患者中验证了这一技术的可行性与耐受度。也是因为这个原因,Moderna 的平台技 术支持其临床 1-3 期实验成功率明显高于行业平均。 其次,AI 技术将为 mRNA 药物研发突破多重壁垒,而中外企业在这一领域处于同一 起跑线,为国内企业提供了弯道超车的机会。中国团队 AI + mRNA 领域的研究也取得显著 进展,对新冠 mRNA 疫苗优化模型的相关研究登上国际顶级期刊《Nature》,显示出国内在该 领域的国际领先实力。 再次,国内由医疗机构发起的 IIT 研究广泛活跃,这种转化医学有望给国内企业带 来更多机遇。IIT 研究不仅加速了科研成果向临床应用的转化,还为国内企业提供了快速的 早期数据验证和实践经验,也可支持潜在的国际合作。4.1 云顶新耀云顶新耀在 mRNA 肿瘤疫苗领域布局广泛,最快已推进至临床阶段。云顶新耀的 mRNA 疗法布局广泛,涵盖个性化肿瘤疫苗、通用疫苗,更是布局了免疫调节肿瘤疫苗以及自体生 成 CAR-T 疗法。其中,个性化肿瘤疫苗 EVM16 已经启动 IIT 研究,并于 2025 年 3 月 6 日在 北京大学肿瘤医院完成首例患者给药; 通用型的现货肿瘤治疗性疫苗 EVM14 在 2025 年 3 月 24 日获 FDA IND 批准,成为其首款自研进入全球临床的 mRNA 肿瘤疫苗。EVER-NEO-1“妙算”AI 平台性能优异。EVM16 是一款云顶新耀自研的新型 mRNA 个性 化肿瘤治疗性疫苗。根据每位患者特有的肿瘤细胞突变,使用自主研发且具备自我迭代能力 的 EVER-NEO-1“妙算”肿瘤新抗原人工智能 AI 算法系统,识别出具有较高免疫原性的肿瘤 新抗原,并设计出编码数十种肿瘤新抗原的 mRNA 治疗性疫苗。“妙算”系统不仅能识别出绝 大多数已被报道的肿瘤新抗原,还识别出了多个之前未报道的肿瘤新抗原,并且在多个独立 验证研究中展现出与行业领先算法相当或更优的新抗原预测能力。临床前数据显示, EVM16 与 PD-1 抗体联用后具有协同抗肿瘤效果,支持个性化肿瘤疫苗与免疫检查点抑制剂在临床 中的联用。4.2 思路迪医药股份思路迪医药的多肽肿瘤疫苗在中美最快已推进至临床后期,mRNA 通用肿瘤疫苗 3D124 即 将进入临床。思路迪医药具备丰富的肿瘤疫苗研发经验,引进的多肽肿瘤疫苗已在全球进入 III 期临床阶段,用于治疗 AML(急性髓系白血病)。根据合作方公告,2024 年 12 月 IDMC(独立第 三方)已给出积极评价,2025 年底将进行最终分析。思路迪负责的国内部分也已经完成了 I 期 安全性数据的收集,有望凭借海外临床的积极数据直接开展注册性桥接试验。3D124 是一款通用 性现货肿瘤疫苗,可靶向多个肿瘤抗原,包括 KRAS、NRAS、EGFR 等,据公司披露,在临床前观 察到较强的抗肿瘤效果,计划在 2025 年向 FDA 及 CDE 提交 NDA 申请。 具备与自家 ICI 恩沃利单抗联用潜力。如前文分析,与 PD-(L)1 等 ICIs 联用有望成为 肿瘤疫苗使用的标准范式,而思路迪医药与拥有一款 PD-L1 单抗恩沃利单抗,可以率先与自家 mRNA 肿瘤疫苗开展联用尝试。恩沃利单抗是一款差异化的皮下注射制剂,通过人源化的单域抗 体结构域 PD-L1 进行结合,给药时长从数小时缩短到 30 秒以内。恩沃利单抗目前获批 MSI-H/dMMR 晚期实体瘤治疗,在胆道癌、非小细胞肺癌等适应症的临床已进行至 III 期。 AI 设计自有 IP 的可电离阳离子脂质体,充实 mRNA 技术平台。2025 年 3 月 11 日公司公 告,公司自主研发的用于核算药物递送的 LNP 关键组分科电力阳离子脂质已申报 PCT。借助人工 智能系统,公司针对不同细胞种类和器官靶向建立了可电离阳离子脂质平台,可以高效协同自研 mRNA 肿瘤疫苗项目的开发,突破技术和专利壁垒,提升差异化优势。4.3 悦康药业全面布局核酸药物领域。2021 年,悦康药业全资收购杭州天龙药业,一举获得了包含 siRNA、ASO、mRNA 在内的多种核酸药物研发平台。mRNA 疫苗方面,公司已有两款产品(YKYY025 和 YKYY026)成功获批中美 IND。其中 YKYY025 针对呼吸道合胞病毒(RSV)引起的下呼吸道疾 病,而 YKYY026 则专注于带状疱疹的预防。 mRNA 技术储备全面。悦康药业的两款 mRNA 预防性疫苗均采用基于完全自主知识产权的 YK-009 阳离子脂质的 LNP 递送系统,递送效率高、安全性好。此外,公司还成功研发了 mRNA 疫 苗冻干技术,并于 2023 年 10 月获得发明专利授权。这项技术突破性地解决了传统 mRNA 疫 苗在非超低温条件下的稳定性问题,大大提高了疫苗的长期储存及运输便利性。 自研肿瘤免疫治疗递送系统,实现高效靶向递送。公司自研的三组分脂质组合物 TLP 能够 高效包载编码特异性抗原 mRNA,并特异性靶向脾脏中抗原呈递细胞,有效促进肿瘤抗原 mRNA 的 摄取、表达和处理,同时提升新抗原的递呈和特异性 T 细胞的激活;此外,TLP 还具有类似 TLR 佐剂功能,可以高效诱导 IFNα、IFNγ、TNFα 等细胞因子的产生,进一步增强 T 细胞活化,在癌 症治疗中具有重要的临床应用意义。4.4 新诺威/石药集团拥有全球第三款、国内首款获准使用的 mRNA 疫苗,唯一经商业化检验的中国药企。石药集团旗下巨石生物自主研发的 COVID-19 mRNA 疫苗度恩泰在 2023 年国家授权紧急使用,成为继 BioNTech、Moderna 后全球第三款、国内首款获准使用的 mRNA 疫苗,巨石生物的 mRNA 疫苗也由 此成为唯一经商业化检验的 mRNA 平台。 完善的 mRNA 技术平台已完成搭建,具备从设计到生产的端到端能力。石药集团已完成搭 建 mRNA 疫苗平台,涵盖了抗原设计、mRNA 疫苗设计、产业化等核心能力,具备安全性和平台拓 展性等核心优势,确保了其在 mRNA 疫苗研发和生产中的高效性和可靠性。借助该平台,SYS6016、 SYS6026、SYS6017 三款分别针对呼吸道合胞病毒(RSV)、人乳头瘤病毒(HPV)和水痘 - 带状 疱疹病毒(VZV)的 mRNA 疫苗在研。 治疗性疫苗方面储备丰富。已将基于 LNP-mRNA 的 BCMA CAR-T 疗法 SYS6020 推向临床,用 于治疗多发性骨髓瘤以及系统性红斑狼疮等多种自免疾病。采用 LNP - mRNA 代替病毒类 DNA 转染,转染效率高,可在体内无扩增,安全性高,且成本较低。4.5 康希诺康希诺是国内创新疫苗龙头,其 mRNA 疫苗技术已获临床数据支持。康希诺生物作为国产 创新疫苗龙头,逐步建立起包括病毒载体疫苗技术、合成疫苗技术、mRNA 技术等新一代疫苗研 发及生产技术平台。其研发的新冠 mRNA 疫苗 CS-2034 完成了多项临床试验,实验证明其安全性 良好,免疫原性佳,且表现出不错的保护率。虽然最终未实现商业化销售,但康希诺验证了其 mRNA 技术平台的价值,并推出 mRNA 多价流感疫苗等后续产品。 合作布局 mRNA 肿瘤疫苗,进入治疗性疫苗新领域。2025 年 4 月 9 日康希诺官宣,已与键 凯科技全资子公司天津键凯科技有限公司签署合作开发协议,双方将共同推进 mRNA 疫苗用于治 疗胶质母细胞瘤(GBM)的临床研究项目,预计今年内完成首例患者给药。在合作中,康希诺生 物提供自有的 mRNA 技术平台,以及拥有自主设计、开发等功能的序列优化软件,可得到影响稳 定性的关键位点及有效提高抗原表达量的最优序列,CMC 工艺简练,可以缩短产品开发时间,快 速实现科研成果产业化。(本文仅供参考,不代表我们的任何投资建议。如需使用相关信息,请参阅报告原文。)识别微信二维码,可添加药时空小编请注明:姓名+研究方向!
信使RNAsiRNA疫苗核酸药物免疫疗法
2025-07-16
·药时空
7月15日,云顶新耀宣布其通用型的现货肿瘤治疗性疫苗EVM14注射液的新药临床试验申请(IND)获国家药品监督管理局药品审评中心(CDE)的正式受理,受理号为CXSL2500584。EVM14是基于云顶新耀自主知识产权的mRNA技术平台研发,是一款靶向5个肿瘤相关抗原的通用型的现货肿瘤治疗性疫苗,拟用于鳞状非小细胞肺癌、头颈鳞癌等瘤种的治疗。临床前试验结果表明,EVM14在小鼠中诱导了剂量依赖性的抗原特异性免疫应答,并在多个小鼠同源肿瘤模型中显著地抑制了肿瘤生长。同时,EVM14能够诱导免疫记忆,展现出有效降低肿瘤复发及转移的能力。此外,研究还证明了EVM14与免疫检查点抑制剂(ICI,如抗PD-1或抗CTLA-4抗体)的联用可以显著增强抗肿瘤活性,支持在临床上对联合用药的探索。2025年3月,EVM14获FDA批准开展临床试验。2025年6月9日,云顶新耀嘉善工厂顺利放行首批GMP临床试验样品,该批样品将用于支持云顶新耀在中美两地开展EVM14的临床试验。合作的临床试验中心包括美国MD Anderson癌症中心和中国上海市胸科医院等。此外,云顶新耀还有一款个性化肿瘤疫苗EVM16。该疫苗已在小鼠模型中验证疗效并与PD-1抗体有协同作用,IIT研究显示即使在低起始剂量下也能在晚期患者中引发良好的免疫原性。该产品目前已在中国启动了一项研究者发起的临床试验,3月4日已完成首例患者给药。图1:云顶新耀在研管线新冠疫情之后,mRNA疫苗在肿瘤治疗领域的潜力逐渐显现,成为未来肿瘤免疫治疗的重要研究方向之一。当前,mRNA肿瘤疫苗的研究主要聚焦肿瘤相关抗原(TAAs)和个性化新抗原(Neoantigens)两类。目前,全球尚未有任何一款mRNA肿瘤疫苗获批上市。不过,Moderna和默沙东联合开发的个性化新抗原疗法mRNA-4157/V940已获得美国食品药品监督管理局(FDA)的突破性疗法认证。Moderna预计该疫苗可能于2027年正式上市。此外,BioNTech开发的mRNA肿瘤疫苗BNT111也在临床开发阶段。至于国内,也有不少企业布局了mRNA肿瘤疫苗。今年年初至今,国产mRNA疫苗企业相继迎来产品研发新进展:1月,北京启辰生生物首款治疗原发性脑胶质母细胞瘤的mRNA-DC肿瘤疫苗正式启动I期临床试验的患者入组工作。3月,云顶新耀个性化肿瘤疫苗EVM16完成首例给药,通用型现货肿瘤治疗性疫苗EVM14获FDA IND批准:星锐医药个性化肿瘤疫苗STR-V005完成首例患者给药。4月,瑞宏迪医药自主研发的国内首款针对恶性实体瘤术后预防复发的个性化新抗原mRNA疫苗RGL-270注射液,正式获得临床试验申请(IND)默示许可;康方生物在Clinicaltrials.gov网站上注册了个体化mRNA疫苗单药或联合PD-1/CTLA-4双抗、PD-1/VEGF双抗辅助治疗胰腺癌的IIT临床试验。康希诺生物与键凯科技签署合作开发协议,双方将共同推进mRNA疫苗用于治疗胶质母细胞瘤(GBM)的临床研究项目,预计今年内完成首例患者给药。6月,新合生物依托AI技术研发的个性化新抗原治疗性mRNA疫苗XH001获CDE批准开展临床试验。......总的来说,随着Moderna、BioNTech等巨头的产品临近上市,以及中国企业的强势突围,全球mRNA肿瘤疫苗的“黄金时代”即将拉开序幕。参考资料:[1] 云顶新耀宣布通用型的现货肿瘤治疗性疫苗EVM14注射液的新药临床试验申请获中国国家药品监督管理局药品审评中心(CDE)正式受理. 云顶新耀. 2025-07-15.[2] 首款 mRNA 肿瘤疫苗上市在即,一览国内超 20 家企业最新研发进展. 医麦客. 2025-05-09.[3] 3月份美国获批!云顶新耀现货肿瘤疫苗国内IND获受理. 药讯随说. 2025-07-16.[4] mRNA癌症疫苗研究进展及中国专利布局(一)(截至2025.6.10). 核药联盟. 2025-06-11.[5] 首款 mRNA 肿瘤疫苗上市在即,中国力量快速崛起. 新浪医药. 2025-05-09.[6] 最快今年上市!mRNA肿瘤疫苗“黄金时代”拉开序幕. 药创新. 2025-05-07.识别微信二维码,可添加药时空小编请注明:姓名+研究方向!
2025-07-14
·药融圈
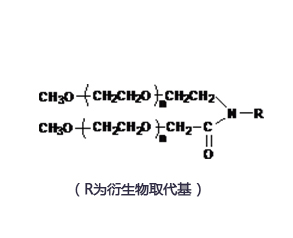
北京键凯科技股份有限公司(股票代码:688356,证券简称:键凯科技)成立于2001年,是一家常年专注于药物递送领域的高新技术企业,2020年8月26日于上交所科创板首发上市。键凯科技是致力于医用药用聚乙二醇衍生物产业化的高新技术企业,是国内外为数不多能进行高纯度和低分散度的医用药用聚乙二醇及活性衍生物工业化生产的公司。键凯科技拥有高纯度PEG原料研制技术平台、医用药用PEG材料平台、PEG医药应用创新平台等3个核心技术平台,实现了从PEG原料、PEG衍生物到PEG修饰药物研制的全面覆盖。目前,公司已在天津开发区西区生物医药园建成占地15000多平方米的医用药用聚乙二醇衍生物的开发及产业化基地,在辽宁省盘锦市精细化工产业园建成了高纯度医用药用聚乙二醇材料的研发与全自动生产线。目前公司及子公司天津键凯科技有限公司、辽宁键凯科技有限公司均获得高新技术企业认证,是国内外为数不多能进行高纯度和低分散度的医用药用聚乙二醇及活性衍生物工业化生产的公司,是国内规模化生产高质量医用药用聚乙二醇及其衍生物领域的先行者之一,也是主要新兴参与者。键凯科技已支持国内7款及海外2款已获批上市的聚乙二醇修饰药物、1款疫苗类药物与国内2款及海外9款已上市的聚乙二醇医疗器械。同时,公司还支持了多家国内国际药企及医疗器械企业的近80个处于临床阶段的项目,产品覆盖600+国内外高校及科研机构。产品与服务全系列聚乙二醇衍生物产品聚乙二醇标准品LNPs递送系统辅料及研发级包封服务脂肪酸侧链系列辅料ADC / PROTAC LINKER客户定制合成服务分析方法开发及质量表征服务临床申报相关质量研究等国家专精特新小巨人企业按照国际cGMP标准生产泛聚乙二醇材料的规模化生产商FDA DMF备案18项,CDE辅料登记7项支持7款国内及3款国际已上市的聚乙二醇化药物支持2款国内及9款国际已上市的凝胶类医疗器械产品已完成200余个产品的cGMP级别的小试与中试天津基地年产可达2吨,单批次生产能力可达50公斤辽宁基地年产量可达12-20吨,单批次生产能力可达100公斤遵循ICHQ7和cGMP准则ISO13485、ISO9001、ISO45001、ISO14001、ISO50001、ISO27001认证键凯支持您的01 聚乙二醇化药物全系列PEG衍生物PEG分子及PEG linker的设计筛选合成路线及分析方法开发临床申报配套质量研究02核酸药物LNPs递送系统辅料创新递送系统组分开发针对核酸药物的分析表征研发级LNP包封服务03多肽类药物全系列PEG衍生物脂肪酸侧链类产品定制化分子偶联服务修饰后多肽及降解产物的表征04凝胶类医疗器械全系列PEG衍生物临床申报配套质量研究定制化凝胶分析方法的开发凝胶用PEG分子及交联剂的设计筛选天津:医用药用聚乙二醇衍生物产业化基地天津市经济技术开发区 占地约15000㎡盘锦:医用聚乙二醇及衍生物产业化与应用成果转化项目辽宁省盘锦市 占地约32000㎡大会详情 /CMC-CHINA点击下方图片了解更多▼
抗体药物偶联物蛋白降解靶向嵌合体疫苗核酸药物引进/卖出
100 项与 北京键凯科技股份有限公司 相关的药物交易
登录后查看更多信息
100 项与 北京键凯科技股份有限公司 相关的转化医学
登录后查看更多信息
组织架构
使用我们的机构树数据加速您的研究。
登录
或

管线布局
2026年03月01日管线快照
管线布局中药物为当前组织机构及其子机构作为药物机构进行统计,早期临床1期并入临床1期,临床1/2期并入临床2期,临床2/3期并入临床3期
药物发现
8
7
临床前
临床3期
1
2
其他
登录后查看更多信息
当前项目
| 药物(靶点) | 适应症 | 全球最高研发状态 |
|---|---|---|
聚乙二醇伊立替康 ( Top I ) | 多形性胶质母细胞瘤 更多 | 临床2期 |
MLPH靶向siRNA(键凯科技) ( MLPH ) | 肿瘤 更多 | 临床前 |
JK-1119I | 肿瘤 更多 | 临床前 |
mRNA vaccine for Glioblastoma(CanSinoBio/Jankem) | 胶质母细胞瘤 更多 | 临床前 |
JK-1214R ( Nav1.9 ) | 镇痛 更多 | 临床前 |
登录后查看更多信息
药物交易
使用我们的药物交易数据加速您的研究。
登录
或

转化医学
使用我们的转化医学数据加速您的研究。
登录
或

营收
使用 Synapse 探索超过 36 万个组织的财务状况。
登录
或

科研基金(NIH)
访问超过 200 万项资助和基金信息,以提升您的研究之旅。
登录
或

投资
深入了解从初创企业到成熟企业的最新公司投资动态。
登录
或

融资
发掘融资趋势以验证和推进您的投资机会。
登录
或

生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用