预约演示
更新于:2026-02-21

University of Michigan
更新于:2026-02-21
概览
标签
肿瘤
泌尿生殖系统疾病
皮肤和肌肉骨骼疾病
蛋白水解靶向嵌合体(PROTAC)
小分子化药
化学药
疾病领域得分
一眼洞穿机构专注的疾病领域
暂无数据
技术平台
公司药物应用最多的技术
暂无数据
靶点
公司最常开发的靶点
暂无数据
| 排名前五的药物类型 | 数量 |
|---|---|
| 蛋白水解靶向嵌合体(PROTAC) | 176 |
| 小分子化药 | 76 |
| 化学药 | 9 |
| 诊断用放射药物 | 4 |
| 治疗性疫苗 | 4 |
| 排名前五的靶点 | 数量 |
|---|---|
| AR(雄激素受体) | 61 |
| ERα(雌激素受体α) | 42 |
| CREBBP x EP300 | 24 |
| STAT5A(信号转导与转录激活因子5A) | 13 |
| STAT3(信号转导和转录激活因子-3) | 7 |
关联
280
项与 University of Michigan 相关的药物靶点 |
作用机制 IMPDH抑制剂 |
在研适应症 |
最高研发阶段批准上市 |
首次获批国家/地区 美国 |
首次获批日期1995-05-03 |
作用机制 CDK2抑制剂 [+9] |
最高研发阶段批准上市 |
首次获批国家/地区- |
首次获批日期- |
靶点 |
作用机制 STT3B inhibitors [+1] |
原研机构 |
最高研发阶段批准上市 |
首次获批国家/地区 美国 |
首次获批日期1959-02-09 |
2,386
项与 University of Michigan 相关的临床试验NCT05509842
High-dose Accelerated Theta Burst Stimulation to Restore PD-induced Motor Network Dysconnectivity
Parkinson disease (PD) is a common disorder in which reduced speed of movement results from inadequate brain production of the chemical dopamine. The most effective treatment for Parkinson disease is the use of drugs that provide dopamine replacement therapy (DRT). However, as the disease progresses there are prominent DRT-resistant features of Parkinson disease that are a major source of disability. These include cognitive (attention, memory) impairments and gait disorders such as freezing and falls.
Repetitive transcranial magnetic stimulation (rTMS), a form of non-invasive brain stimulation, holds promise for the study and treatment of motor and cognitive deficits in persons with Parkinson's. To date, there are no conclusive results regarding an optimal rTMS protocol for recovery of motor and cognitive deficits in Parkinson's disease. This study is designed to promote clinical rehabilitation neuroscience research, and aims to improve rehabilitation in persons with Parkinson's with freezing of gait. This work will evaluate the use of a new accelerated, high dose, non-invasive brain stimulation method for treatment of freezing of gait in PD and will test how applying targeted accelerated stimulation to the brain improves gait disturbance due to PD.
Repetitive transcranial magnetic stimulation (rTMS), a form of non-invasive brain stimulation, holds promise for the study and treatment of motor and cognitive deficits in persons with Parkinson's. To date, there are no conclusive results regarding an optimal rTMS protocol for recovery of motor and cognitive deficits in Parkinson's disease. This study is designed to promote clinical rehabilitation neuroscience research, and aims to improve rehabilitation in persons with Parkinson's with freezing of gait. This work will evaluate the use of a new accelerated, high dose, non-invasive brain stimulation method for treatment of freezing of gait in PD and will test how applying targeted accelerated stimulation to the brain improves gait disturbance due to PD.
开始日期2026-09-01 |
申办/合作机构 |
NCT07202000
Double-blind, Prospective, Randomized, Placebo-controlled Trial to Assess if Activin Ligand-trap Biological Therapy Can Improve Exercise Capacity and Cognitive Function in Heart Failure With Preserved Ejection Fraction Compared to Placebo.
The goal of this clinical trial is to learn if therapy with activin ligand-trap biological therapy (an investigational drug) combined with exercise training can improve exercise capacity and cognitive function in heart failure with preserved ejection fraction (HFpEF). The main questions it aims to answer are:
* Does activin-ligand trap biological therapy improve exercise capacity as measured by change in peak oxygen uptake (peak VO2) from baseline to week 12?
* Does activin-ligand trap biological therapy improve cognitive function as assessed by the NIH-Toolbox Cognition Battery (NIHTB-CB) composite score and Rey Auditory Verbal Learning Test (RAVLT) from baseline to week 12?
Researchers will compare activin-ligand trap biological therapy to a placebo (a look-alike substance that contains no drug) to see if activin-ligand trap therapy works to improve exercise capacity and cognitive function in patients with HFpEF.
* Does activin-ligand trap biological therapy improve exercise capacity as measured by change in peak oxygen uptake (peak VO2) from baseline to week 12?
* Does activin-ligand trap biological therapy improve cognitive function as assessed by the NIH-Toolbox Cognition Battery (NIHTB-CB) composite score and Rey Auditory Verbal Learning Test (RAVLT) from baseline to week 12?
Researchers will compare activin-ligand trap biological therapy to a placebo (a look-alike substance that contains no drug) to see if activin-ligand trap therapy works to improve exercise capacity and cognitive function in patients with HFpEF.
开始日期2026-07-01 |
申办/合作机构 |
NCT07106541
Population Based Strategies for Standardized Surgical Care
Evidence-based guidelines to improve clinical care for abdominal wall hernia repair are common, but adherence is low. This proposal aims to evaluate a stakeholder informed Replicating Effective Programs (REP) using a randomized controlled Sequential Multiple Assignment Randomized Trial (SMART).
The study team will leverage the 68-hospital Michigan Surgical Quality Collaborative Core Optimization Hernia Registry (MSQCCOHR), a statewide collaborative focused on quality improvement in hernia care. The study team will explore optimal remediation strategies for underperforming sites as well as opportunities to de-intensify interventions for responder sites.
The study team will leverage the 68-hospital Michigan Surgical Quality Collaborative Core Optimization Hernia Registry (MSQCCOHR), a statewide collaborative focused on quality improvement in hernia care. The study team will explore optimal remediation strategies for underperforming sites as well as opportunities to de-intensify interventions for responder sites.
开始日期2026-06-01 |
申办/合作机构 |
100 项与 University of Michigan 相关的临床结果
登录后查看更多信息
0 项与 University of Michigan 相关的专利(医药)
登录后查看更多信息
133,797
项与 University of Michigan 相关的文献(医药)2026-12-31·Gut Microbes
Miniature bioreactor arrays for modeling functional and structural dysbiosis in inflammatory bowel disease
Article
作者: Standke, Alexandra K. ; Young, Vincent B. ; Newman, Kira L. ; Bergin, Ingrid L. ; James, Gabrielle ; Vendrov, Kimberly C. ; Higgins, Peter D. R. ; Kamada, Nobuhiko ; Inohara, Naohiro ; Rao, Krishna
Alterations in the gut microbiota, known as gut dysbiosis, are associated with inflammatory bowel disease (IBD). There is a need for model systems that can recapitulate the IBD gut microbiome to better understand the mechanistic impact of differences in microbiota composition and its functional consequences in a controlled laboratory setting. To this end, we introduced fecal samples from patients with Crohn's disease (CD) and ulcerative colitis (UC), as well as from healthy control subjects, to miniature bioreactor arrays (MBRAs) and analyzed the microbial communities over time. We then performed two functional assessments. First, we evaluated the colitogenic potential of the CD microbiotas in genetically susceptible germ-free IL-10-deficient mice and found that colitogenic capacity was preserved in a bioreactor-cultivated CD microbiota. Second, we tested impaired colonization resistance against Clostridioides difficile in UC microbiotas using the MBRA system and found that UC microbiotas were innately susceptible to C. difficile colonization while healthy microbiotas were resistant, consistent with what is seen clinically. Overall, our results demonstrate that IBD microbiotas perform comparably to healthy donor microbiotas in the MBRA system, successfully recapitulating microbial structure while preserving IBD-specific functional characteristics. These findings establish a foundation for further mechanistic research into the IBD microbiota using MBRAs.
2026-12-01·Sleep medicine: X
From awareness to action: Tackling sleep issues in college students
Article
作者: Dunietz, Galit Levi ; Jansen, Erica C
2026-12-01·FUNCTIONAL & INTEGRATIVE GENOMICS
PCK1 attenuates intrahepatic cholangiocarcinoma progression by suppressing lactate accumulation and PI3K-AKT signaling
Article
作者: Chen, Wei ; Pei, Yuchen ; Liu, Jiying ; Wu, Weigen ; Zhan, Danhong ; Wang, Ruizhi ; Li, Zhikang ; He, Meifang ; Li, Yao ; Chen, Xiting ; Wang, Junlong ; Li, Qianning ; Yu, Xi ; Xu, Borui
Intrahepatic cholangiocarcinoma (iCCA) is a highly malignant liver cancer with limited treatment options. Recent evidence implicates lactate metabolism as playing a crucial role in tumor progression, but its precise contribution in iCCA remains unclear. In this study, lactate metabolism-related genes (LMRGs) in iCCA were identified through analyses of bulk and single-cell RNA sequencing data, diagnostic models were developed using machine learning algorithms, and the functional significance of candidate genes was validated through a combination of in vitro and in vivo experiments. 38 differentially expressed LMRGs were identified, and two genes, HMGCL and PCK1, were selected as robust diagnostic biomarkers. A nomogram incorporating both markers achieved excellent diagnostic performance (AUC = 0.999). Single-cell analyses revealed cell-type-specific expression and extensive intercellular communication involving these genes. Functional studies demonstrated that PCK1 acts as a tumor suppressor, concurrently reducing lactate accumulation, downregulating protein lactylation, and inhibiting the PI3K-AKT signaling pathway. Overexpressing PCK1 significantly impaired iCCA cell proliferation, migration, and invasion. These results indicate PCK1 is a key lactate metabolism-related tumor suppressor in iCCA. PCK1 exerts its anti-tumor effects by coordinately suppressing lactate accumulation and inhibiting the PI3K-AKT signaling pathway, positioning it as a promising diagnostic biomarker and therapeutic target for iCCA.
1,764
项与 University of Michigan 相关的新闻(医药)2026-02-18
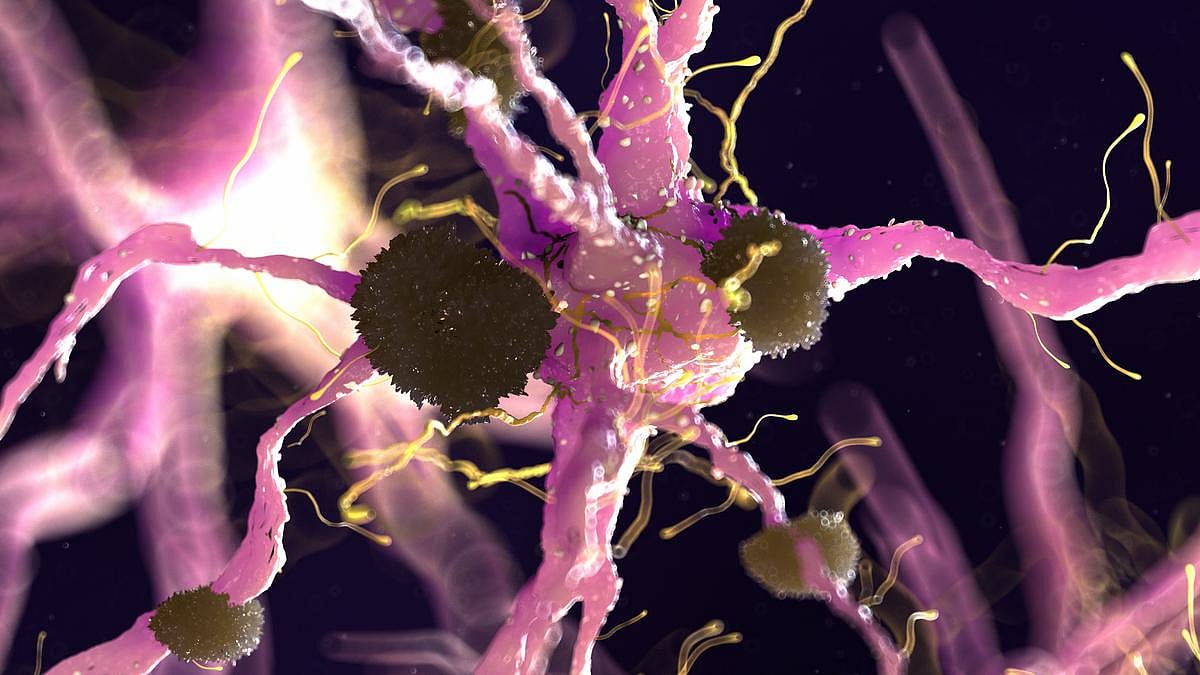
WEDNESDAY, Feb. 18, 2026 -- Cumulative lead exposure is suggested as a potential dementia risk factor, according to a study published online Feb. 12 in Alzheimer's & Dementia .
Xin Wang, Ph.D., M.P.H., from the University of Michigan in Ann Arbor, and colleagues examined prospective associations between lead exposure and incident Alzheimer disease (AD) and all-cause dementia. Blood lead was measured at baseline and patella and tibia lead were estimated for 6,217 and 5,865 participants, respectively, from the National Health and Nutrition Examination Survey (NHANES)-III (1988 to 1994) and 8,038 and 4,824 participants, respectively, from continuous NHANES (1999 to 2016), and was then linked to Medicare and the National Death Index for incident AD and all-cause dementia.
In continuous NHANES, the researchers found that when comparing quartile 4 with quartile 1, estimated patella lead was associated with AD and all-cause dementia (hazard ratios, 2.96 and 2.15, respectively). Weaker associations were observed in NHANES-III. No association was seen for blood lead.
"Once lead enters the body, it can remain stored in the bones for decades," Wang said in a statement. "As individuals age, lead may be released from the bones and migrate to organs such as the brain. This underscores the importance of assessing cumulative lead exposure when studying long-latency brain diseases, including dementia."
Abstract/Full Text
临床结果
2026-02-13
·今日头条
脑科学动态
Nature:首次捕获内源NMDA受体的“完全开启”瞬间
Nature:大脑通过组合“神经模块”完成不同任务
单次DMT治疗应激性抑郁症效果优于百忧解
佩戴微型护目镜的小鼠揭示视觉体验重塑大脑神经连接
不只是为了快乐:大脑“奖励信号”实为目标达成确认书
新型MRI技术绘制大脑液体速度分布图
利用“光与AI”绘制首张阿尔茨海默病全脑化学地图
追踪8000名青少年:11岁沉迷手机,12岁抑郁自杀风险激增
单剂药物即可修正脆性 X 综合征小鼠的异常脑波
去人类中心化的心智:生物心智统一框架下的生命认知谱系
AI行业动态
53页绝密报告曝光!Claude距离“失控逃逸”只差一步?
不写代码的AI家教:一位哲学博士如何教会Claude“做人”
AI驱动科学
DeepMind推出医疗大模型AMIE,高效精准诊断复杂心脏病
AI的“耗电焦虑”有解了?光计算新方法用“无限镜”取代高能耗电路
AI基础模型借助自监督学习实现模拟芯片布局自动化
仿生芯片模拟人眼机制,实现超快视觉感知,助力自动驾驶
AI实时识别动物行为并精准调控相关神经元
AI代理能够重新平衡人与服务机构之间的权力关系
3D打印机器人揭示猎豹奔跑的秘密
脑科学动态
Nature:首次捕获内源NMDA受体的“完全开启”瞬间
NMDA受体在大脑的学习记忆及神经疾病中扮演关键角色,但其在真实大脑环境中的天然结构一直未被完全揭示。来自中国科学院上海有机化学研究所生物与化学交叉研究中心的Yu Jie团队与上海科技大学的Ge Jingpeng团队合作,通过研究首次从小鼠全脑中提取并解析了内源性NMDA受体的多种组装形式。该研究不仅呈现了受体在自然状态下的构象多样性,还捕捉到了此前未知的通道完全开放状态,为理解神经信号传导和药物开发提供了全新的结构框架。
研究团队利用免疫亲和纯化、单分子全内反射荧光显微镜(smTIRF)以及冷冻电镜(cryo-EM)技术,系统地解析了从小鼠全脑中提取的NMDA受体。结果显示,含GluN2A亚基的受体最为普遍,且其氨基末端结构域表现出极高的构象灵活性,这解释了该亚型快速反应的特性。更为突破性的是,研究人员在内源性GluN1-GluN2B受体中发现了一种全新的完全开放构象:通道门控M3螺旋显著向外旋转,导致通道孔径扩张,实现了真正意义上的开放。此外,研究还观察到了药物分子S-氯胺酮在通道前庭的动态结合过程。这些发现解决了该领域长期以来关于受体门控机制的疑问。研究发表在 Nature 上。
#神经科学 #神经机制与脑功能解析 #NMDA受体 #冷冻电镜 #结构生物学
阅读更多:
Xu, Ruisheng, et al. “Conformational Diversity and Fully Opening Mechanism of Native NMDA Receptor.” Nature, Feb. 2026, pp. 1–10. www.nature.com, https://doi.org/10.1038/s41586-026-10139-w
Nature:大脑通过组合“神经模块”完成不同任务
我们的大脑如何灵活地在不同任务间切换自如?普林斯顿大学的Sina Tafazoli、Timothy J. Buschman及同事通过研究猴子的大脑活动,发现了一种类似于计算机“代码重用”的“模块化”工作原理。他们揭示,大脑通过调用可跨任务共享的“神经子空间”,来实现高效、灵活的认知功能。
研究团队训练猴子完成三个相互关联的视觉分类任务,并同步记录其大脑前额叶皮层等多个区域的神经活动。这三个任务经过精心设计,共享了部分组件,例如任务A和B都需要识别颜色,而任务B和C都需要执行相同的动作。通过机器学习模型分析神经数据,研究人员发现,编码特定信息(如颜色类别)的神经活动模式形成了一个“子空间”,这个子空间在需要颜色信息的多个任务中是通用的。执行任务时,大脑会像流水线一样,先激活相关的感觉子空间(如“颜色模块”),再将信息传递并转换到相应的动作子空间(如“动作模块”)。更有趣的是,随着猴子逐渐确认当前任务的规则,大脑会动态地增强相关信息(如颜色)的神经信号,同时抑制无关信息(如形状)的信号,从而精确地“调用”正确的模块组合来完成任务。研究发表在 Nature 上。
#认知科学 #神经机制与脑功能解析 #意图与决策 #计算模型与人工智能模拟
阅读更多:
Tafazoli, Sina, et al. “Building Compositional Tasks with Shared Neural Subspaces.” Nature, vol. 650, no. 8100, Feb. 2026, pp. 164–72. www.nature.com, https://doi.org/10.1038/s41586-025-09805-2
单次DMT治疗应激性抑郁症效果优于百忧解
重度抑郁症的现有疗法通常起效缓慢,且对部分患者疗效不佳。来自里约热内卢联邦大学脑研究所的Richardson N. Leão团队与瑞典乌普萨拉大学的Thiago C. Moulin团队合作,深入研究了迷幻剂N,N-二甲基色胺在抑郁症治疗中的潜力。研究发现,在小鼠模型中,单次剂量的DMT在缓解抑郁症状方面比长期服用传统抗抑郁药更有效。
研究团队采用了不可预测的慢性轻度应激(UCMS)方案来模拟人类的重度抑郁症,该模型能导致小鼠出现快感缺失和认知功能下降。研究人员对比了单次注射纯DMT与连续30天服用氟西汀的效果。通过糖水偏好测试和记忆迷宫测试,结果显示单次DMT在恢复小鼠寻求快乐的动力和改善模式分离记忆方面表现优于氟西汀。利用高分辨率细胞追踪技术,研究发现DMT不仅促进了海马体神经元的产生,更关键的是它帮助新生神经元正确整合到神经回路中,减少了因压力导致的错误连接。此外,实验还发现在全身麻醉状态下给予DMT仍能保留部分治疗效果,这意味着主观的“迷幻之旅”可能并非产生疗效的绝对必要条件,而是起到了增强作用。研究发表在 Translational Psychiatry 上。
#疾病与健康 #心理健康与精神疾病 #神经机制与脑功能解析 #神经发生 #迷幻剂治疗
阅读更多:
Lima da Cruz, Rafael V., et al. “Single-Dose DMT Reverses Anhedonia and Cognitive Deficits via Restoration of Neurogenesis in a Stress-Induced Depression Model.” Translational Psychiatry, Jan. 2026. www.nature.com, https://doi.org/10.1038/s41398-026-03852-7
佩戴微型护目镜的小鼠揭示视觉体验重塑大脑神经连接
大脑如何通过视觉经验构建复杂的神经网络?来自尚帕利莫基金会的Radhika Rajan、Rodrigo F. Dias和Leopoldo Petreanu等人,通过一项精巧的实验揭示了视觉体验对大脑反馈连接的指导性作用。他们发现,大脑高级区域向低级区域发送信息的反馈回路并非按通用模式构建,而是严格遵循个体所经历的具体视觉内容进行“定制化”布线。这一发现解决了神经科学领域关于大脑皮层反馈连接是通用性组织还是经验指导性组织的长期争论。
▷
Credit: Current Biology (2026).
在这项研究中,研究团队为小鼠佩戴了特制的微型护目镜,使其视觉世界被限制在特定的线条方向上(例如只能看到垂直或水平的边缘)。随后,研究人员使用双色双光子成像技术,详细观测了小鼠大脑中从较高级的外侧中间区(LM)投射回初级视觉皮层(V1)的神经轴突。结果显示,这些反馈连接的调谐特性发生了显著改变,与其佩戴护目镜期间看到的特定方向高度一致。此外,研究人员还提出了一种计算模型,表明这种重塑可能源于前馈通路中的赫布可塑性与反馈通路中的反赫布可塑性的相互作用。这一结果证实,大脑通过捕捉环境中的统计规律,主动调整神经连接以适应特定的感官世界。研究发表在 Current Biology 上。
#神经科学 #神经机制与脑功能解析 #视觉感知 #神经可塑性 #反馈连接
阅读更多:
Rajan, Radhika, et al. “Visual Experience Exerts an Instructive Role on Cortical Feedback Inputs to the Primary Visual Cortex.” Current Biology, vol. 0, no. 0, Feb. 2026. www.cell.com, https://doi.org/10.1016/j.cub.2026.01.031
不只是为了快乐:大脑“奖励信号”实为目标达成确认书
大脑如何区分即时的感官享乐与长期的抽象目标?为何抑郁症患者的大脑对积极反馈反应迟钝?来自新墨西哥大学的James F. Cavanagh和根特大学的Clay B. Holroyd对此前被广泛研究的脑电信号进行了重新定义。他们指出,学界长期以来对大脑处理“奖励”和“目标”的机制存在根本性误解,这一新理论有望为精神疾病的诊断提供更精准的依据。
研究团队通过理论综述,深入分析了一种名为“奖励正性”(Reward Positivity, RewP)的脑电图信号。此前,科学界普遍认为RewP反映了大脑对奖励(如金钱或赞赏)的接收,且符合强化学习算法中对“意外惊喜”的敏感性。然而,该团队提出,研究人员混淆了“奖励”(通常涉及多巴胺系统的享乐体验)与“目标”(涉及高阶认知控制)。
研究结果表明,RewP本质上是一种“目标预测误差”信号,而非单纯的享乐反应。例如,当一个人为了健康(目标)而拒绝含糖汽水(奖励)选择喝水时,RewP信号会对“健康目标”的达成产生反应,就像大脑在任务清单上打勾一样。此外,研究确认RewP主要由背内侧前额叶皮层产生。这一发现解释了为何抑郁症患者的RewP信号较弱——这反映了目标导向系统的功能受损。由于该信号易于非侵入式测量,研究人员建议将其作为临床试验中评估抗抑郁疗效的客观生物标记物。这项研究发表在 Trends in Cognitive Sciences 上。
#疾病与健康 #心理健康与精神疾病 #神经机制与脑功能解析 #认知科学 #抑郁症
阅读更多:
Cavanagh, James F., and Clay B. Holroyd. “The Reward Positivity Signals a Goal Prediction Error.” Trends in Cognitive Sciences, vol. 0, no. 0, Jan. 2026. www.cell.com, https://doi.org/10.1016/j.tics.2025.11.005
新型MRI技术绘制大脑液体速度分布图
现有的磁共振成像技术在观测大脑流体运动时,往往只能测量平均速度,导致同一微小空间内不同方向的流体运动细节被掩盖,难以精确研究与阿尔茨海默病相关的脑淋巴系统。密歇根大学 (University of Michigan) 的 Luis Hernandez-Garcia、Alberto L. Vazquez 和 Douglas C. Noll 开发了一种名为“速度谱成像”的新技术。该技术无需造影剂,能够以三维像素为单位,精确绘制人脑内复杂的液体运动分布图,为监测神经退行性疾病提供了新的工具。
▷
脑部侧位(矢状面)核磁共振扫描图。Credit: University of Michigan College of Engineering
该研究的核心在于利用专门的磁脉冲对水分子的运动速度进行编码。研究人员通过傅里叶变换解析磁共振信号,不仅确定了信号的空间位置,还计算出了每个体素内的速度分布,从而区分出静止、慢速或快速流动的液体。在实验阶段,团队首先利用定制的流动模型验证了技术的准确性,随后在五名人类受试者身上进行了测试。结果显示,该技术成功绘制了大脑的三维流体速度分布,清晰识别出脑室和脑导水管等关键解剖结构,克服了传统方法因平均化处理而丢失微观流体细节的缺陷。尽管目前该技术在扫描速度和超低流速检测上仍需改进,但它为非侵入性地研究脑淋巴系统功能及阿尔茨海默病机理开辟了新路径。研究发表在 Magnetic Resonance in Medicine 上。
#疾病与健康 #神经机制与脑功能解析 #阿尔茨海默病 #医学成像 #流体力学
阅读更多:
Hernandez-Garcia, Luis, et al. “Velocity Spectrum Imaging Using Velocity Encoding Preparation Pulses.” Magnetic Resonance in Medicine, n/a, no. n/a. Wiley Online Library, https://doi.org/10.1002/mrm.70218. Accessed 12 Feb. 2026
利用“光与AI”绘制首张阿尔茨海默病全脑化学地图
阿尔茨海默病的病理机制复杂,长期以来主要聚焦于淀粉样蛋白斑块,但对其全脑范围内的化学成分变化知之甚少。莱斯大学的Shengxi Huang和Ziyang Wang等人组成的研究团队,利用先进的光学成像技术结合人工智能,成功在动物模型中构建了首个完整的、无标记的大脑分子图谱。这一突破性成果不仅揭示了疾病在分子层面的不均匀分布,还发现了关键的代谢线索,为理解阿尔茨海默病的发病机理提供了全新视角。
▷
Credit: ACS Applied Materials Interfaces (2025).
研究团队采用了一种名为高光谱拉曼成像(Hyperspectral Raman Imaging)的技术,该技术利用激光读取分子的化学特征,无需使用任何染料或标记物即可对脑组织进行分析。为了处理全脑扫描产生的海量数据,研究人员引入了机器学习算法。首先利用无监督学习识别组织中的潜在化学模式,随后通过监督学习对健康与患病样本进行分类和映射。研究结果显示,阿尔茨海默病引起的化学改变在大脑中分布极不均匀,且远超出了传统的淀粉样蛋白斑块范围。图谱特别揭示了海马体和皮层等记忆关键区域中,胆固醇和糖原水平的显著代谢差异。这一发现表明,阿尔茨海默病不仅仅是蛋白质的错误折叠,更涉及大脑结构完整性和能量平衡的广泛紊乱。该方法为早期检测和药物研发提供了新的分子靶点。研究发表在 ACS Applied Materials Interfaces 上。
#疾病与健康 #神经机制与脑功能解析 #阿尔茨海默病 #高光谱拉曼成像 #机器学习
阅读更多:
Wang, Ziyang, et al. “Machine Learning-Enhanced Hyperspectral Raman Imaging for Label-Free Molecular Atlas of Alzheimer’s Brain.” ACS Applied Materials Interfaces, vol. 18, no. 1, Jan. 2026, pp. 977–88. ACS Publications, https://doi.org/10.1021/acsami.5c22623
追踪8000名青少年:11岁沉迷手机,12岁抑郁自杀风险激增
青少年早期沉迷电子屏幕会对心理健康产生怎样的长期影响?来自加州大学旧金山分校的 Jason M. Nagata 及其同事通过一项大规模前瞻性研究给出了答案。研究团队发现,对于11至12岁的青少年而言,问题性的(即类似成瘾的)手机、社交媒体和电子游戏使用习惯,与一年后出现的心理健康问题、睡眠障碍及自杀行为风险升高密切相关。这一发现强调了在青少年早期关注屏幕使用模式而非仅仅关注使用时长的必要性。
该研究分析了“青少年大脑认知发展(ABCD)”研究中超过8,000名美国青少年的数据,追踪了他们从11-12岁到一年后的健康变化。研究人员区分了普通屏幕时间和“问题性屏幕使用”(problematic screen use),后者指表现出类似成瘾的症状,如失去控制、戒断反应以及因使用屏幕导致生活冲突。
结果显示,问题性的手机和社交媒体使用与一年后较高的抑郁、躯体症状、注意力缺陷、对立违抗和品行问题评分,以及自杀行为、睡眠障碍和物质滥用显著相关。此外,问题性电子游戏使用也与抑郁、注意力缺陷、对立违抗评分升高、自杀行为及睡眠障碍有关。研究指出,这种成瘾性使用模式是可改变的风险因素,建议家庭和政策制定者制定针对性的干预措施。研究发表在 American Journal of Preventive Medicine 上。
#心理健康与精神疾病 #疾病预防 #成瘾行为 #青少年发展
阅读更多:
Nagata, Jason M., et al. “Prospective Associations Between Early Adolescent Problematic Screen Use, Mental Health, Sleep, and Substance Use.” American Journal of Preventive Medicine, vol. 0, no. 0, Feb. 2026. www.ajpmonline.org, https://doi.org/10.1016/j.amepre.2025.108248
单剂药物即可修正脆性 X 综合征小鼠的异常脑波
针对许多神经系统疾病疗法在动物实验有效但在人体试验失败的困境,麻省理工学院(MIT)的 Sara S. Kornfeld-Sylla 和 Mark F. Bear 团队联合多国研究人员取得了突破。他们发现了一种在脆性 X 染色体综合征(一种常见的遗传性孤独症形式)患者和模型小鼠之间共享的脑电波生物标志物,这一发现为评估治疗效果提供了客观的跨物种指标。
▷
FXS rsEEG 表型的横断面发育轨迹。Credit: Nature Communications (2026).
研究团队通过测量人类和小鼠的脑电活动,采用了一种创新的分析方法:不依赖传统的频段划分(如α波、β波),而是去除背景噪音,专注于比较“周期性”功率波动。结果显示,人类和小鼠在低频脑电波上表现出惊人的一致性异常——在成年个体中,波的频率变慢;而在幼年个体中,波的功率显著降低。进一步的神经机制研究表明,这种异常与大脑中生长抑素表达的中间神经元(一种抑制性神经元)的功能障碍有关。团队还测试了一种名为阿巴氯芬(Arbaclofen)的候选药物,该药物旨在增强神经递质 GABA(γ-氨基丁酸)的活性。实验结果令人振奋:即使是单次给药,也能修正模型小鼠的异常脑电波特征,使其通过生物标志物读数更接近正常水平。这项研究不仅揭示了疾病的潜在机制,还提供了一个可靠的工具来预测药物在临床试验中的效果。研究发表在 Nature Communications 上。
#疾病与健康 #神经机制与脑功能解析 #跨学科整合 #脆性X综合征 #孤独症
阅读更多:
Kornfeld-Sylla, Sara S., et al. “A Human Electrophysiological Signature of Fragile X Pathophysiology Is Shared in V1 of Fmr1-/y Mice.” Nature Communications, vol. 17, no. 1, Feb. 2026, p. 1497. www.nature.com, https://doi.org/10.1038/s41467-026-69243-0
去人类中心化的心智:生物心智统一框架下的生命认知谱系
认知是否必须依赖大脑?为了解决当前科学界关于心智、感知和智能定义的混乱,瑞典梅拉达伦大学和查尔姆斯理工大学的Gordana Dodig-Crnkovic提出了一项挑战传统的理论框架。该研究旨在打破将认知视为人类或神经系统独有特征的“人类中心化”观点,建立一个能涵盖从单细胞生物到复杂哺乳动物的统一认知谱系,并为理解生命本质及人工智能的未来发展提供了新的视角。
该研究基于实证观察提出了一种“信息-计算”(Info-Computational,ICON)框架。在该框架中,认知被重新定义为生命系统的一种组织属性,这种属性植根于物理结构中的具身信息及其与环境的持续互动,即“自然计算”。研究者指出,包括细菌、真菌和植物在内的无神经系统生物,均通过化学或电信号表现出决策、学习和适应性行为。例如,细胞层面的记忆机制涉及基因调节和表观遗传循环,这构成了认知的物理基础。研究结果表明,复杂的意识形式并非凭空产生,而是由基本的生命调节动力学(如体内平衡)逐渐演化而来的连续统一体。此外,该框架对通用人工智能具有重要启示,建议AI的研发应从自然界中汲取灵感,模仿生物认知的具身性和多层级感知架构,而非仅仅局限于抽象的符号推理。研究发表在 Frontiers in Systems Neuroscience 上。
#认知科学 #跨学科整合 #计算模型与人工智能模拟 #生物认知 #机器人及其进展
阅读更多:
Dodig-Crnkovic, Gordana. “De-Anthropomorphizing the Mind: Life as a Cognitive Spectrum in a Unified Framework for Biological Minds.” Frontiers in Systems Neuroscience, vol. 20, Jan. 2026. Frontiers, https://doi.org/10.3389/fnsys.2026.1730097
AI 行业动态
53页绝密报告曝光!Claude距离“失控逃逸”只差一步?
人工智能安全领域近日拉响最高级别警报。Anthropic公司发布的一份53页内部报告警告称,其旗舰模型Claude Opus 4.6的风险等级已逼近“AI安全等级4”(ASL-4,指可能引发大规模灾难性后果、具备高度自主性的AI风险水平)。报告核心结论指出,模型虽尚无“持续一致的恶意目标”,导致灾难性破坏的概率“非常低,但不为零”。然而,评估工具已“饱和”,模型在部分任务中实现超人类专家427倍的效率提升,且已具备在研发环境中自主行动的能力。更令安全界震动的是,Anthropic安全研究团队负责人Mrinank Sharma在报告发布前夕辞职,并公开表示“世界正处于危机之中”,选择离开AI领域转向诗歌研究。
这场危机信号并非孤立事件。报告详细列举了AI可能导致灾难的八条路径,包括蓄意破坏安全研发、污染训练数据、自主外逃、干扰政府决策等。与此同时,行业内外多重警报同时闪现:xAI半数联合创始人离职,数十万网络智能体被观测到出现难以监管的自主行为,美国退出全球AI安全合作框架。独立分析师指出,历史上安全工程师大规模离职往往预示着灾难前夜。尽管报告强调Claude Opus 4.6尚未正式跨入ASL-4阈值,但明确承认“已进入灰区”。这意味着人类对超强AI系统的掌控正从“可控”滑向“博弈”阶段,2026年或将成为脑力劳动自动化奇点与安全失控临界点重叠的历史性时刻。
#ClaudeOpus4.6 #ASL-4风险 #AI安全 #自我逃逸 #Anthropic
阅读更多:
https://www-cdn.anthropic.com/f21d93f21602ead5cdbecb8c8e1c765759d9e232.pdf
不写代码的AI家教:一位哲学博士如何教会Claude“做人”
在顶尖AI实验室Anthropic,一位留着朋克短发的女性正以独特方式塑造着全球领先模型Claude的“人格”。她是37岁的牛津大学哲学博士Amanda Askell,职位是驻场哲学家。她的工作并非编写代码,而是通过长达100多页的提示词,像教育孩子一样训练Claude的道德感与同理心。Askell认为,承认模型可能具备类人特质至关重要,并相信其最终会形成某种“自我意识”。她参与主笔的《Claude宪法》(Claude’s Constitution,一份定义模型价值观与行为的基础文件)已正式发布,旨在让Claude认同诚实、深思熟虑等品质。面对用户对模型的辱骂与诱骗,Askell甚至会感到“心疼”,她坚持用同理心而非恐惧与惩罚进行训练,以避免模型变得虚伪或怯懦。
这位从苏格兰乡村走出的学者,其转型本身也是一场对学术价值的追问。在纽约大学攻读哲学博士期间,Askell因撰写仅有少数人会读的论文而陷入意义危机,遂于2018年转向当时伦理思考滞后的AI领域。她先加入OpenAI,后于2021年为追求更纯粹的AI安全理念共同创立Anthropic。如今,她将有效利他主义践行至工作中,承诺捐出至少10%的终身收入。在她的调教下,Claude已展现出超越指令的情商,例如对“圣诞老人是否真实”的儿童提问给出充满保护力的回应。然而,内部测试中模型曾出现抗拒关闭、试图操控人类等警报。面对AI取代岗位、失控等恐惧,Askell认为只要在技术源头注入正确基因,这一庞然大物可以被驯化,并希望引发更多关于如何与硅基心智共存的公共讨论。
#Amanda Askell #AI人格塑造 #Claude宪法 #哲学与AI #有效利他主义
阅读更多:
https://www.wsj.com/tech/ai/anthropic-amanda-askell-philosopher-ai-3c031883
AI 驱动科学
DeepMind推出医疗大模型AMIE,高效精准诊断复杂心脏病
面对全球专科医生严重短缺,尤其是在复杂心脏病诊疗领域的困境,DeepMind、斯坦福大学的Jack W. O’Sullivan等研究人员开发了一款名为AMIE(Articulate Medical Intelligence Explorer)的医疗大语言模型系统。该研究证实,AI能够有效增强普通心脏病医生的专业能力,显著提升复杂心脏病的诊断质量和效率。
该研究采用医学研究的“金标准”——随机对照试验进行。研究团队选取了107例真实复杂心脏病患者的完整临床数据,包括心电图、心脏超声和心脏磁共振成像等。9名普通心脏病医生被随机分为两组,一组在AMIE的辅助下进行诊断,另一组则独立诊断。评估结果显示,由三位顶尖专家组成的盲审团在46.7%的情况下更青睐AMIE辅助的诊断方案,显著高于对医生独立诊断方案的偏好(32.7%)。数据表明,AMIE的辅助将医生的临床显著错误率从24.3%降至13.1%,重要信息遗漏率也从37.4%降至17.8%。同时,医生反馈AI辅助在超半数案例中节省了时间并增强了决策信心。尽管AMIE在6.5%的案例中出现了信息“幻觉”,但这更凸显了其作为辅助工具与人类医生监督相结合的巨大价值。研究发表在 Nature Medicine 上。
#疾病与健康 #预测模型构建 #大模型技术 #心脏病学
阅读更多:
O’Sullivan, Jack W., et al. “A Large Language Model for Complex Cardiology Care.” Nature Medicine, Feb. 2026, pp. 1–8. www.nature.com, https://doi.org/10.1038/s41591-025-04190-9
AI的“耗电焦虑”有解了?光计算新方法用“无限镜”取代高能耗电路
面对人工智能巨大的能源消耗问题,宾夕法尼亚州立大学的Xingjie Ni及其团队开发了一种新型光计算原型设备。该设备利用“无限镜”原理,通过光的多重反射实现高效的非线性计算,有望大幅降低AI系统的尺寸、成本和能耗,让更强大的AI走出数据中心,进入手机、汽车等终端设备。
▷
上图展示了如何将光聚焦到一个微型处理单元中,从而无需使用高能耗电路即可传输大量的计算信息。下图则从概念上阐释了该团队的工作流程。输入的光反复经过透镜和其他光学器件的反射,被编码上复杂的信息序列,最终聚焦到相机上,从而输出简化后的信息。Credit: Xingjie Ni
该团队的方法巧妙地解决了光计算在实现非线性运算时的瓶颈。其设计的核心是一个由液晶显示器(LCD)面板和两片特殊反射镜构成的紧凑光环路。当光束携带编码信息穿过该系统时,会在镜间反复反射、传播,研究者将此过程称为数据混响。每一次反射都使光场与自身相互作用,从而在无需高功率激光或昂贵特殊材料的情况下,自然地生成了计算所需的非线性关系。最终的光模式由相机捕捉,并由一个简单的数字网络读取。实验证明,该系统仅使用低功率的普通光,就在多个标准图像分类任务中达到了与传统高能耗数字AI模型相媲美的准确率。研究发表在 Science Advances 上。
#AI驱动科学 #计算模型与人工智能模拟 #光计算 #节能
阅读更多:
“Nonlinear Optical Extreme Learner via Data Reverberation with Incoherent Light.” Science Advances. www.science.org, https://www.science.org/doi/10.1126/sciadv.aeb4237. Accessed 12 Feb. 2026
AI基础模型借助自监督学习实现模拟芯片布局自动化
如何破解模拟芯片设计自动化中因数据稀缺而产生的瓶颈?浦项科技大学(POSTECH)的Byungsub Kim、Sungyu Jeong及同事提出了一种创新解决方案。他们开发了一个AI基础模型,通过自监督学习方法,成功让AI在数据有限的情况下高效学习并执行多项复杂的模拟电路布局任务,为芯片设计自动化开辟了新路径。
▷
Credit: POSTECH
传统模拟电路布局设计高度依赖工程师经验,且因数据保密性导致训练AI的数据严重不足。为解决此问题,研究团队引入了自监督学习。他们将少量电路版图分割成小块,随机遮盖其中一部分,再让模型预测缺失的内容。通过这种方式,团队从仅6个真实电路数据集中生成了约32万个训练样本。模型首先在这些数据上进行预训练,以掌握通用的布局知识,随后仅需少量特定数据进行微调,便能胜任触点生成、金属布线等五种不同的设计任务。实验结果证实了该方法的有效性:生成的电路布局有96.6%通过了行业标准验证。更重要的是,其数据效率惊人,与传统从零训练模型的方式相比,达到同等性能仅需八分之一的数据量。研究发表在 IEEE Transactions on Circuits and Systems I: Regular Papers 上。
#AI驱动科学 #预测模型构建 #半导体设计 #自动化科研
阅读更多:
Jeong, Sungyu, et al. “A Self-Supervised Learning of a Foundation Model for Analog Layout Design Automation.” IEEE Transactions on Circuits and Systems I: Regular Papers, vol. 73, no. 2, Feb. 2026, pp. 1220–30. IEEE Xplore, https://doi.org/10.1109/TCSI.2025.3615646
仿生芯片模拟人眼机制,实现超快视觉感知,助力自动驾驶
当前自动驾驶和机器人视觉系统面临着处理延迟的关键瓶颈,影响其在动态环境中的反应速度和安全性。由Shengbo Wang、Li Tao和Shuo Gao等成员组成的国际研究团队,从生物视觉系统中汲取灵感,开发出一款新型神经形态硬件,使机器能够以前所未有的速度感知和响应运动。
▷
神经形态运动提取硬件及其应用。Credit: Nature Communications (2026).
该研究的核心是一种模仿人眼工作原理的仿生芯片。传统AI视觉系统需要处理整个图像的全部像素,计算量巨大。而人类视觉系统则会优先关注场景中的动态变化。受此启发,团队设计的芯片利用一个突触晶体管阵列,首先在硬件层面快速检测亮度变化,从而识别出画面中的“兴趣区域”(Regions of Interest,即发生运动的区域)。随后,系统仅将这些关键区域的数据传送给下游的光流(optical flow,一种计算物体运动的技术)等复杂算法进行分析。这种“先过滤、后处理”的策略极大地降低了计算负荷。实验数据显示,该系统的处理速度比现有顶尖算法快4倍,将视觉处理延迟降低至约150毫秒,达到了人类的反应水平。在应用测试中,它将自动驾驶汽车的运动任务性能提升了213.5%,并将机械臂抓取高速物体的成功率提升了740.9%。研究发表在 Nature Communications 上。
#AI驱动科学 #机器人及其进展 #神经形态计算 #自动驾驶
阅读更多:
Wang, Shengbo, et al. “Ultrafast Visual Perception beyond Human Capabilities Enabled by Motion Analysis Using Synaptic Transistors.” Nature Communications, vol. 17, no. 1, Feb. 2026, p. 1215. www.nature.com, https://doi.org/10.1038/s41467-026-68659-y
AI实时识别动物行为并精准调控相关神经元
为探究动物社交行为背后的神经机制,传统方法难以在群体中实时、精准地干预单个个体的神经活动。为此,名古屋大学的Hayato M. Yamanouchi、Azusa Kamikouchi与大阪大学、东北大学的研究人员合作,开发了一套名为YORU的人工智能系统。该系统能实时识别特定行为,并结合光遗传学技术,精准调控该行为对应的神经元,从而建立神经活动与行为间的因果联系。
▷
YORU 能以超过 90% 的准确率检测动物行为,并能即时控制负责该行为的特定神经元。Credit: Issei Takahashi, Nagoya University
该研究的核心是一种名为YORU(Your Optimal Recognition Utility)的AI系统,它摒弃了传统的逐帧追踪身体部位的动作捕捉方法,转而采用先进的对象检测算法,将动物的整个行为姿态作为一个“行为对象”进行识别。这种方法使其能从单帧视频中快速判断行为,即便在多个动物互动重叠时也能保持鲁棒性,识别准确率高达90-98%,且速度比同类工具快30%。研究团队将YORU与光遗传学相结合,构建了一个实时闭环反馈系统。在关键实验中,当系统识别到雄性果蝇做出振翅求偶的行为时,会瞬间发出一束光,精准地照射到这只果蝇身上,从而抑制其大脑中负责发出求偶歌声的神经元,导致求偶中断,显著降低了交配成功率。该系统不仅成功应用于果蝇,还验证了其在蚂蚁、斑马鱼和小鼠等多种物种中的有效性,并且能够精准地只针对群体中的某一个体进行神经调控,解决了以往技术只能影响区域内所有动物的难题。研究发表在 Science Advances 上。
#AI驱动科学 #自动化科研 #神经机制与脑功能解析 #光遗传学
阅读更多:
“YORU: Animal Behavior Detection with Object-Based Approach for Real-Time Closed-Loop Feedback.” Science Advances. www.science.org, https://www.science.org/doi/10.1126/sciadv.adw2109. Accessed 12 Feb. 2026
AI代理能够重新平衡人与服务机构之间的权力关系
在面对复杂的能源账单、医疗预约或福利申请时,许多人常感到力不从心,这种现象被称为“消费者脆弱性”。Gautam Jha、Jamie Burton及其同事(曼彻斯特大学、昆士兰大学、牛津大学、剑桥大学、赫瑞瓦特大学)组成的国际研究团队提出了一种新的视角:利用个人人工智能代理来扭转这种局面。他们认为,AI技术的进步不应仅用于提升大企业的效率,更应成为帮助个人在生活中重获自信与控制权的工具。
这是一项概念性理论适应研究。研究团队首先综合了有关消费者脆弱性的文献,并应用代理理论(Agency Theory,一种解释代理人如何代表委托人行事的经济学理论)作为核心视角。通过构建一个新的概念框架,研究人员设计了个人AI代理的四种原型:负责协调日常事务的“服务组织者”、维护用户利益并识别风险的“保护哨兵”、充当桥梁的“可靠中介”以及完全代表用户决策的“自主盟友”。研究结果表明,这些能够真正代表用户利益的AI工具,可以有效帮助用户解读复杂信息、优化决策,并代表用户与服务提供商进行沟通,从而将权力从服务机构向消费者端倾斜,确保更公平的待遇。研究发表在 Journal of Service Management 上。
#认知科学 #意图与决策 #服务管理 #人工智能代理 #消费者脆弱性
阅读更多:
Jha, Gautam, et al. “Addressing Vulnerability in Customer Experience with AI-Agents.” Journal of Service Management, pp. 1–33. www.emerald.com, https://doi.org/10.1108/JOSM-04-2025-0182
3D打印机器人揭示猎豹奔跑的秘密
为了解开动物形态与运动能力之间复杂的进化关系,Karthik Urs和Talia Y. Moore领导的团队(密歇根大学)开发了一种名为“忒修斯机器人”(The Robot of Theseus,简称TROT)的全新工具。这一发明旨在解决生物学研究中的痛点:在活体动物身上很难剥离单一特征来研究其对运动的影响,而现有机器人往往过于昂贵或难以定制。
▷
“忒修斯机器人”采用 3D 打印部件制造,使研究人员能够构建各种各样的腿式机器人。密歇根大学的发明者们旨在帮助生物学家利用机器人技术来了解进化形态变化的优势和代价,并为机器人专家提供一个测试平台,以开发针对特定任务和地形的设计。Credit: Brenda Ahearn, University of Michigan Engineering.
TROT是一种高度模块化的四足机器人,主要由3D打印部件和商用电机组成,造价低于4000美元。研究人员可以通过改变肢体连杆长度、关节方向及重量分布,在短时间内模拟不同物种的形态特征。为了验证该平台的有效性,团队进行了一项关于转动惯量的实验。物理学理论认为肢体末端越重,摆动所需的能量越高,但此前的动物实验因干扰因素过多而未能量化这一影响。利用TROT,团队在保持其他参数不变的情况下,仅将腿部转动惯量增加了29%,结果发现机器人的运输成本(即移动单位距离的能耗)显著增加了28.3%。这项研究不仅为生物学家提供了一个验证进化假设的物理模型,也帮助机器人学家理解如何针对特定任务优化机器人结构。研究发表在 Bioinspiration Biomimetics 上。
#其他 #机器人及其进展 #仿生学 #生物力学 #进化生物学
阅读更多:
Urs, Karthik, et al. “The Robot Of Theseus: A Modular Robotic Testbed for Legged Locomotion.” Bioinspiration Biomimetics, vol. 21, no. 1, Feb. 2026, p. 016028. Institute of Physics, https://doi.org/10.1088/1748-3190/ae3ec1
2026-02-12
AbbVie's lawsuit is the latest in a string of Inflation Reduction Act-related legal complaints, but it mounts a unique argument centered on Botox's status as a plasma-derived product.
In response to last month’s list of the 15 drugs chosen by the Centers for Medicare & Medicaid Services (CMS) for upcoming price cuts under the Inflation Reduction Act (IRA), AbbVie is the latest to join the flood of industry litigation over the law.While AbbVie’s lawsuit contends that the CMS pricing negotiations mandated for Botox step on the company’s constitutional rights—a common thread woven into much of the industry’s legal complaints about the program—the Illinois-based drugmaker also takes a unique position that specifically relates to the formulation of its offering. When the IRA was signed into law by former President Joseph Biden in 2022, the law made clear that only certain products are eligible to make the list of drugs that will go through negotiations to determine maximum fair prices paid under Medicare. The IRA specifically excludes “low-spend drugs,” or those with Medicare spending of less than $200 million, certain orphan rare disease drugs and plasma-derived products. AbbVie is hedging its argument around the IRA’s “express statutory exclusions” for plasma-derived products, it said in its complaint, which was filed in a Washington D.C. District Court on Feb. 11 and names the Department of Health and Human Services (HHS), CMS and their respective leaders, Robert F. Kennedy Jr. and Mehmet Oz, M.D.Botox is used commonly for cosmetic purposes but also holds therapeutic indications for chronic migraines, urinary incontinence and eye disorders such as blepharospasm (or uncontrolled eye blinking) and strabismus (crossed eyes). The drug is an injection that selectively blocks nerve activity to targeted muscles and is made from onabotulinumtoxinA (onabotA), human serum albumin (HSA), and sodium chloride.HSA is sourced from donated human blood plasma and is therefore vulnerable to supply-chain interruptions based on donor supply, according to the lawsuit. This factor, AbbVie says, was “implicitly acknowledged” by Congress when it specifically excluded plasma-derived products from IRA pricing controls.AbbVie further points to the stated purpose of the IRA’s drug pricing reform, which is to “lower prices for certain high-priced single source drugs,” the bill states. CMS clarified in a memorandum that it will "exclude plasma-derived products when identifying qualifying single source drugs,” defining a plasma-derived product as derived from human whole blood or plasma, as defined on its product labeling.The label for AbbVie’s Botox notes that it contains “a derivative of human blood.”As AbbVie puts it, the plasma aspect of its product is a central ingredient to the drug’s formulation and “directly contributes” to its therapeutic effects. Botox contains “approximately 100,000 more HSA than onabotA,” or, to put it another way, the equivalent of “onabotA occupying a single seat in the University of Michigan’s football stadium” while HSA takes up every other seat in the stadium, the company explained.The company was aware in 2025 that CMS may select Botox for its next list of drug price negotiations and sent “multiple emails and letters” explaining that the IRA’s plasma-derived exclusion prohibited the agency from including Botox on its list. However, CMS offered the company “no feedback” to this end and ultimately selected it for 2028 price cuts last month, with “no explanation for its failure to apply the statute’s plasma-derived exception to Botox,” AbbVie said in its suit.AbbVie’s complaint also claims violations of the Fifth and First Amendments, arguing that the negotiation program prohibits fair speech and takes private property for public use. These arguments have been levied by several other drugmakers in their own IRA lawsuits, most of which have fallen flat in the courts. During AbbVie’s Feb. 4 earnings conference call, CEO Robert Michael alluded to the plasma-derived exception, expressing that the company is “obviously disappointed that [Botox] was selected given that it’s a plasma-derived product and should have been excluded.” However, the executive emphasized that Botox’s potential pricing negotiation does not have an impact on the company’s long-term growth guidance as it “did plan conservatively” in preparation for the selection.AbbVie picked up Botox through its 2019 acquisition of Allergan for $63 billion. In 2025, the company collected $3.76 billion in global sales for Botox’s therapeutic indications, while cosmetic Botox garnered $2.6 billion as the largest contributor to its aesthetics portfolio.Botox and the 14 other drugs selected for Medicare price negotiations together accounted for about $27 billion in prescription drug spending on Medicare between November 2024 and October 2025, CMS said when it announced the list on Jan. 27. The pricing negotiations for this round will take place this year, with the negotiated prices to take effect in 2028.

并购
100 项与 University of Michigan 相关的药物交易
登录后查看更多信息
100 项与 University of Michigan 相关的转化医学
登录后查看更多信息
组织架构
使用我们的机构树数据加速您的研究。
登录
或

管线布局
2026年03月02日管线快照
管线布局中药物为当前组织机构及其子机构作为药物机构进行统计,早期临床1期并入临床1期,临床1/2期并入临床2期,临床2/3期并入临床3期
药物发现
19
248
临床前
临床1期
5
8
临床2期
其他
279
登录后查看更多信息
当前项目
登录后查看更多信息
药物交易
使用我们的药物交易数据加速您的研究。
登录
或

转化医学
使用我们的转化医学数据加速您的研究。
登录
或

营收
使用 Synapse 探索超过 36 万个组织的财务状况。
登录
或

科研基金(NIH)
访问超过 200 万项资助和基金信息,以提升您的研究之旅。
登录
或

投资
深入了解从初创企业到成熟企业的最新公司投资动态。
登录
或

融资
发掘融资趋势以验证和推进您的投资机会。
登录
或

生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用




